Abstract
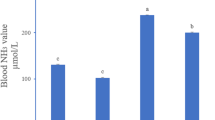
The aim of the present study was to investigate the effect of selenium (Se) deficiency on the relationship between the pyroptosis and MAPK signaling pathway in spleen injury. A total of 10 two-month-old Sus scrofa domesticus specimens were allocated to two groups. The control group was fed a basal diet (0.15-mg/kg Se), and the experimental group was fed a 0.03-mg/kg Se-deficient diet for 2 months. The pig-spleen histopathological changes were observed with hematoxylin–eosin staining. Frozen sections were prepared to detect the content of ROS in pig-spleen cells. The oxidation stress related indexes were determined using a spectrophotometer. The levels of pyroptosis- and MAPK signaling pathway-related factors were detected via quantitative real-time polymerase chain reaction (qPCR) and western blotting (WB). The results of sections showed that the lymphocytes decreased in number, the spacing of cells widened, and some cells were necrotic in the spleen tissue of pigs fed a low-selenium diet. The content of ROS, malondialdehyde, nitric oxide, H2O2, and catalase activity in the low-selenium group was significantly higher than that in the control group, and SOD activity was decreased. The protein-ratio-levels of p-Nrf2 to Nrf2 were decreased. The expression levels of nod-like receptor (NLR) family pyrin domain containing 3 (NLRP3), IL-1β, IL-18, ASC, gasdermin D, and caspase-1, the protein-ratio-levels of p-AKT serine/threonine kinase (p-AKT) to AKT, p-extracellular regulated protein kinases (ERK) to ERK, p-P38 MAPK to p-P38, and p–c-Jun N-terminal kinase (p-JNK) to JNK were significantly increased in the Se-deficient group compared with the control group. These results suggested that Se deficiency can induce oxidant stress, which increases pyroptosis- and inflammation-related factors of pig-spleen injury.




Similar content being viewed by others
References
Gać P, Czerwińska K, Macek P, Jaremków A, Mazur G, Pawlas K, Poręba R (2021) The importance of selenium and zinc deficiency in cardiovascular disorders. Environ Toxicol Pharmacol 82:103553. https://doi.org/10.1016/j.etap.2020.103553
Wang N, Tan HY, Li S, Xu Y, Guo W, Feng Y (2017) Supplementation of micronutrient selenium in metabolic diseases: its role as an antioxidant. Oxid Med Cell Longev 2017:7478523. https://doi.org/10.1155/2017/7478523
Rayman MP (2012) Selenium and human health. Lancet 379(9822):1256–1268. https://doi.org/10.1016/s0140-6736(11)61452-9
Holben DH, Smith AM (1999) The diverse role of selenium within selenoproteins: a review. J Am Diet Assoc 99(7):836–843. https://doi.org/10.1016/s0002-8223(99)00198-4
Rosen BP, Liu Z (2009) Transport pathways for arsenic and selenium: a minireview. Environ Int 35(3):512–515. https://doi.org/10.1016/j.envint.2008.07.023
Papp LV, Lu J, Holmgren A, Khanna KK (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9(7):775–806. https://doi.org/10.1089/ars.2007.1528
Ding D, Mou D, Zhao L, Jiang X, Che L, Fang Z, Xu S et al (2021) Maternal organic selenium supplementation alleviates LPS induced inflammation, autophagy and ER stress in the thymus and spleen of offspring piglets by improving the expression of selenoproteins. Food Funct 12(22):11214–11228. https://doi.org/10.1039/d1fo01653a
Zhang R, Qi J, Zhou M, Pan T, Zhang Z, Yao Y, Han H, Han Y (2021) Upregulation of Nrf2 attenuates oxidative stress-induced, complement activation-associated endothelial injury and apoptosis in transplant-associated thrombotic microangiopathy. Transplant Cell Ther 27(9):758.e751-758.e758. https://doi.org/10.1016/j.jtct.2021.06.017
Thiruvengadam M, Venkidasamy B, Subramanian U, Samynathan R, Ali Shariati M, Rebezov M, Girish S, Thangavel S, et al (2021) Bioactive compounds in oxidative stress-mediated diseases: targeting the NRF2/ARE signaling pathway and epigenetic regulation. Antioxidants (Basel) 10(12). https://doi.org/10.3390/antiox10121859
Su L, Zhang J, Gomez H, Kellum JA, Peng Z (2022) Mitochondria ROS and mitophagy in acute kidney injury. Autophagy 1–14. https://doi.org/10.1080/15548627.2022.2084862
Xian H, Watari K, Sanchez-Lopez E, Offenberger J, Onyuru J, Sampath H, Ying W et al (2022) Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity 55(8):1370-1385.e1378. https://doi.org/10.1016/j.immuni.2022.06.007
Wei P, Yang F, Zheng Q, Tang W, Li J (2019) The potential role of the NLRP3 inflammasome activation as a link between mitochondria ROS generation and neuroinflammation in postoperative cognitive dysfunction. Front Cell Neurosci 13:73. https://doi.org/10.3389/fncel.2019.00073
He Y, Hara H, Núñez G (2016) Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 41(12):1012–1021. https://doi.org/10.1016/j.tibs.2016.09.002
Martinon F, Burns K, Tschopp J (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell 10(2):417–426. https://doi.org/10.1016/s1097-2765(02)00599-3
Lei Q, Yi T, Chen C (2018) NF-κB-Gasdermin D (GSDMD) axis couples oxidative stress and NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome-mediated cardiomyocyte pyroptosis following myocardial infarction. Med Sci Monit 24:6044–6052. https://doi.org/10.12659/msm.908529
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y et al (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526(7575):660–665. https://doi.org/10.1038/nature15514
He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J (2015) Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res 25(12):1285–1298. https://doi.org/10.1038/cr.2015.139
Zhang Z, Zhang Y, Xia S, Kong Q, Li S, Liu X, Junqueira C et al (2020) Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579(7799):415–420. https://doi.org/10.1038/s41586-020-2071-9
Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y, Wang Y et al (2020) Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 368(6494). https://doi.org/10.1126/science.aaz7548
Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F (2017) Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547(7661):99–103. https://doi.org/10.1038/nature22393
Wang Q, Wang Y, Ding J, Wang C, Zhou X, Gao W, Huang H et al (2020) A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature 579(7799):421–426. https://doi.org/10.1038/s41586-020-2079-1
Li S, Sun W, Zhang K, Zhu J, Jia X, Guo X, Zhao Q et al (2021) Selenium deficiency induces spleen pathological changes in pigs by decreasing selenoprotein expression, evoking oxidative stress, and activating inflammation and apoptosis. J Anim Sci Biotechnol 12(1):65. https://doi.org/10.1186/s40104-021-00587-x
Li S, Sun Y, Song M, Song Y, Fang Y, Zhang Q, Li X et al (2021) NLRP3/caspase-1/GSDMD-mediated pyroptosis exerts a crucial role in astrocyte pathological injury in mouse model of depression. JCI Insight 6(23):e146852. https://doi.org/10.1172/jci.insight.146852
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270(5240):1326–1331. https://doi.org/10.1126/science.270.5240.1326
Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME (1999) Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286(5443):1358–1362. https://doi.org/10.1126/science.286.5443.1358
Zhang Z, Zhang H, Li D, Zhou X, Qin Q, Zhang Q (2021) Caspase-3-mediated GSDME induced Pyroptosis in breast cancer cells through the ROS/JNK signalling pathway. J Cell Mol Med 25(17):8159–8168. https://doi.org/10.1111/jcmm.16574
Matsumoto T, Turesson I, Book M, Gerwins P, Claesson-Welsh L (2002) p38 MAP kinase negatively regulates endothelial cell survival, proliferation, and differentiation in FGF-2-stimulated angiogenesis. J Cell Biol 156(1):149–160. https://doi.org/10.1083/jcb.200103096
Stefani C, Miricescu D, Stanescu S II, Nica RI, Greabu M, Totan AR, Jinga M (2021) Growth factors, PI3K/AKT/mTOR and MAPK signaling pathways in colorectal cancer pathogenesis: where are we now? Int J Mol Sci 22:10260. https://doi.org/10.3390/ijms221910260
Ulasov AV, Rosenkranz AA, Georgiev GP, Sobolev AS (2022) Nrf2/Keap1/ARE signaling: Towards specific regulation. Life Sci 291:120111. https://doi.org/10.1016/j.lfs.2021.120111
Shen C, Wang J, Feng M, Peng J, Du X, Chu H, Chen X (2021) The mitochondrial-derived peptide MOTS-c attenuates oxidative stress injury and the inflammatory response of H9c2 cells through the Nrf2/ARE and NF-κB pathways. Cardiovasc Eng Technol. https://doi.org/10.1007/s13239-021-00589-w
Ran Y, Su W (2021) Curcumin ameliorates white matter injury after ischemic stroke by inhibiting microglia/macrophage pyroptosis through NF-κB suppression and NLRP3 inflammasome inhibition. Oxid Med Cell Longev 2021:1552127. https://doi.org/10.1155/2021/1552127
Zheng Y, Zhang B, Guan H, Jiao X, Yang J, Cai J, Liu Q et al (2021) Selenium deficiency causes apoptosis through endoplasmic reticulum stress in swine small intestine. BioFactors 47:788–800. https://doi.org/10.1002/biof.1762
Zhang C, Wang X, Nie G, Wei Z, Pi S, Wang C, Yang F et al (2021) In vivo assessment of molybdenum and cadmium co-induce nephrotoxicity via NLRP3/Caspase-1-mediated pyroptosis in ducks. J Inorg Biochem 224:111584. https://doi.org/10.1016/j.jinorgbio.2021.111584
Funding
This work was supported by the Natural Science Foundation of Henan Province (202300410008) and the Natural Science Foundation of China (32102744).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Lichao Song, Zhihui Jiang, Xingwang Zhang, and Yuwei Song. The first draft of the manuscript was written by Lichao Song, Zhihui Jiang, and Guodong Wang, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The institution of Animal Protection and Utilization Committee at Anyang Institute of Technology approved this experiment.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, L., Jiang, Z., Zhang, X. et al. Selenium Deficiency via the ROS/NLRP3/IL-1β Signaling Pathway Leads to Pyroptosis Injury in Pig Spleen. Biol Trace Elem Res 201, 5192–5200 (2023). https://doi.org/10.1007/s12011-023-03595-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03595-2