Abstract
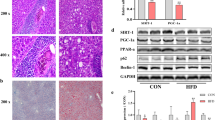
Metabolic-associated fatty liver disease (MAFLD) (previously known as nonalcoholic fatty liver disease (NAFLD)) is a disease with high worldwide prevalence, but with limited available therapeutic interventions. Autophagy is a cell survival mechanism for clearing excess lipids in hepatocytes and affects the occurrence and development of MAFLD. In addition, some studies have shown that magnesium deficiency is common in patients with obesity and metabolic syndrome. Magnesium supplementation can effectively improve metabolism-related diseases such as obesity and fatty liver. Our study successfully constructed a cellular model of MAFLD by 1 mM free fatty acid (FFA) intervention in LO2 cells for 24 h, and there was an increase in lipid accumulation in hepatocytes after FFA intervention. Magnesium supplementation was shown to reduce lipid deposition in hepatocytes induced by FFA, and Western blotting (WB) analysis showed that magnesium supplementation could downregulate the expression of Fasn and SREBP1 and increase the expression of LPL, suggesting that magnesium can reduce lipid accumulation by reducing lipid synthesis and increasing lipid oxidation. Magnesium supplementation could affect cellular lipid metabolism by activating the AMPK/mTOR pathway to stimulate autophagy. Our results identified a relationship between magnesium and lipid accumulation in hepatocytes and showed that magnesium supplementation reduced lipid deposition in hepatocytes by activating autophagy by activating the AMPK-mTOR pathway.






Similar content being viewed by others
Data availability
The authors state the availability of necessary data from the corresponding author upon reasonable request.
References
Arab J, Arrese M, Trauner M (2018) Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu Rev Pathol 13:321–350. https://doi.org/10.1146/annurev-pathol-020117-043617
Adams L, Lymp J, St Sauver J, Sanderson S, Lindor K, Feldstein A, Angulo P (2005) The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129:113–121. https://doi.org/10.1053/j.gastro.2005.04.014
White D, Kanwal F, El-Serag H (2012) Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol : the Official Clin practice J American Gastroenterol Ass 10:1342-1359.e1342. https://doi.org/10.1016/j.cgh.2012.10.001
Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Jarvinen H, Fan JG, Gronbaek H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J (2020) A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol 73:202–209. https://doi.org/10.1016/j.jhep.2020.03.039
Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, Zheng MH, Shiha G, Yilmaz Y, Gani R, Alam S, Dan YY, Kao JH, Hamid S, Cua IH, Chan WK, Payawal D, Tan SS, Tanwandee T, Adams LA, Kumar M, Omata M, George J (2020) The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int 14:889–919. https://doi.org/10.1007/s12072-020-10094-2
Huang DQ, El-Serag HB, Loomba R (2021) Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 18:223–238. https://doi.org/10.1038/s41575-020-00381-6
Elin RJ (1994) Magnesium: the fifth but forgotten electrolyte. Am J Clin Pathol 102:616–622. https://doi.org/10.1093/ajcp/102.5.616
Flatman PW (1984) Magnesium transport across cell membranes. J Membr Biol 80:1–14. https://doi.org/10.1007/BF01868686
Hartwig A (2001) Role of magnesium in genomic stability. Mutat Res 475:113–121. https://doi.org/10.1016/s0027-5107(01)00074-4
Piuri G, Zocchi M, Della Porta M, Ficara V, Manoni M, Zuccotti GV, Pinotti L, Maier JA, Cazzola R (2021) Magnesium in obesity, metabolic syndrome, and type 2 diabetes. Nutrients 13(2):320. https://doi.org/10.3390/nu13020320
Lu L, Chen C, Yang K, Zhu J, Xun P, Shikany JM, He K (2020) Magnesium intake is inversely associated with risk of obesity in a 30-year prospective follow-up study among American young adults. Eur J Nutr 59:3745–3753. https://doi.org/10.1007/s00394-020-02206-3
Ravn HB, Korsholm TL, Falk E (2001) Oral magnesium supplementation induces favorable antiatherogenic changes in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 21:858–862. https://doi.org/10.1161/01.atv.21.5.858
Yue J, Jin S, Gu S, Sun R, Liang Q (2019) High concentration magnesium inhibits extracellular matrix calcification and protects articular cartilage via Erk/autophagy pathway. J Cell Physiol 234:23190–23201. https://doi.org/10.1002/jcp.28885
Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, Bugianesi E, Sirlin CB, Neuschwander-Tetri BA, Rinella ME (2015) Nonalcoholic fatty liver disease. Nat Rev Dis Primers 1:15080. https://doi.org/10.1038/nrdp.2015.80
Martin S, Parton RG (2006) Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol 7:373–378. https://doi.org/10.1038/nrm1912
Scorletti E, Carr RM (2022) A new perspective on NAFLD: focusing on lipid droplets. J Hepatol 76:934–945. https://doi.org/10.1016/j.jhep.2021.11.009
Zechner R, Strauss JG, Haemmerle G, Lass A, Zimmermann R (2005) Lipolysis: pathway under construction. Curr Opin Lipidol 16:333–340. https://doi.org/10.1097/01.mol.0000169354.20395.1c
Finn PF, Dice JF (2006) Proteolytic and lipolytic responses to starvation. Nutrition 22:830–844. https://doi.org/10.1016/j.nut.2006.04.008
Cui W, Sathyanarayan A, Lopresti M, Aghajan M, Chen C, Mashek DG (2021) Lipophagy-derived fatty acids undergo extracellular efflux via lysosomal exocytosis. Autophagy 17:690–705. https://doi.org/10.1080/15548627.2020.1728097
Yang Z, Klionsky DJ (2010) Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol 22:124–131. https://doi.org/10.1016/j.ceb.2009.11.014
Wang Y, Chen C, Chen J, Sang T, Peng H, Lin X, Zhao Q, Chen S, Eling T, Wang X (2022) Overexpression of NAG-1/GDF15 prevents hepatic steatosis through inhibiting oxidative stress-mediated dsDNA release and AIM2 inflammasome activation. Redox Biol 52:102322. https://doi.org/10.1016/j.redox.2022.102322
Wang S, Kuang X, Fang Z, Huang Z, Shi P (2014) Effect of oleic acid on the levels of eight metal ions in human hepatoma SMMC-7721 cells. Biol Trace Elem Res 159:445–450. https://doi.org/10.1007/s12011-014-0018-4
Kurstjens S, van Diepen JA, Overmars-Bos C, Alkema W, Bindels RJM, Ashcroft FM, Tack CJJ, Hoenderop JGJ, de Baaij JHF (2018) Magnesium deficiency prevents high-fat-diet-induced obesity in mice. Diabetologia 61:2030–2042. https://doi.org/10.1007/s00125-018-4680-5
Dai B, Li X, Xu J, Zhu Y, Huang L, Tong W, Yao H, Chow DH, Qin L (2021) Synergistic effects of magnesium ions and simvastatin on attenuation of high-fat diet-induced bone loss. Bioact Mater 6:2511–2522. https://doi.org/10.1016/j.bioactmat.2021.01.027
Su M, Cao D, WangZ, DuanY, HuangY (2021) Fatty acid synthase inhibitor platensimycin intervenes the development of nonalcoholic fatty liver disease in a mouse model. Biomedicines 10(1):5. https://doi.org/10.3390/biomedicines10010005
Han J, Li E, Chen L, Zhang Y, Wei F, Liu J, Deng H, Wang Y (2015) The CREB coactivator CRTC2 controls hepatic lipid metabolism by regulating SREBP1. Nature 524:243–246. https://doi.org/10.1038/nature14557
Ding L, Sun W, Balaz M, He A, Klug M, Wieland S, Caiazzo R, Raverdy V, Pattou F, Lefebvre P, Lodhi IJ, Staels B, Heim M, Wolfrum C (2021) Peroxisomal beta-oxidation acts as a sensor for intracellular fatty acids and regulates lipolysis. Nat Metab 3:1648–1661. https://doi.org/10.1038/s42255-021-00489-2
Li J, Li L, Guo D, Li S, Zeng Y, Liu C, Fu R, Huang M, Xie W (2020) Triglyceride metabolism and angiopoietin-like proteins in lipoprotein lipase regulation. Clin Chim Acta 503:19–34. https://doi.org/10.1016/j.cca.2019.12.029
Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ (2009) Autophagy regulates lipid metabolism. Nature 458:1131–1135. https://doi.org/10.1038/nature07976
Codogno P, Mehrpour M, Proikas-Cezanne T (2011) Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nat Rev Mol Cell Biol 13:7–12. https://doi.org/10.1038/nrm3249
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149:274–293. https://doi.org/10.1016/j.cell.2012.03.017
Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ, Oh KS, Koh EH, Won JC, Kim MS, Oh GT, Yoon M, Lee KU, Park JY (2006) AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem Biophys Res Commun 340:291–295. https://doi.org/10.1016/j.bbrc.2005.12.011
Sun L, Zhang S, Yu C, Pan Z, Liu Y, Zhao J, Wang X, Yun F, Zhao H, Yan S, Yuan Y, Wang D, Ding X, Liu G, Li W, Zhao X, Liu Z, Li Y (2015) Hydrogen sulfide reduces serum triglyceride by activating liver autophagy via the AMPK-mTOR pathway. Am J Physiol Endocrinol Metab 309:E925-935. https://doi.org/10.1152/ajpendo.00294.2015
Feeney KA, Hansen LL, Putker MC (2016) Olivares-Yanez, J. Day, L.J. Eades, L.F. Larrondo, N.P. Hoyle, J.S. O’Neill, G. van Ooijen. Daily magnesium fluxes regulate cellular timekeeping and energy balance, Nature 532:375–379. https://doi.org/10.1038/nature17407
Funding
This study was supported by the National Natural Science Foundation of China (NSFC) (No. 81700716), the Medical Science and Technology Research Foundation of Guangdong Province (No. A2022347), Science and Technology Planning Project in the field of social development of Zhuhai of Guangdong Province (No. 2220004000269), and the Nature Science Foundation of Guangdong Province. (No. 2022A1515011244).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The idea design and coordination of the research were jointly completed by Yingjuan Zeng and Jian Li. Material preparation, data collection, and analysis were performed by Shiyan Chen, Shunkui Lou, Jianhui Xie, and Baojia Zou. The first draft of the manuscript was written by Shiyan Chen and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, S., Luo, S., Zou, B. et al. Magnesium Supplementation Stimulates Autophagy to Reduce Lipid Accumulation in Hepatocytes via the AMPK/mTOR Pathway. Biol Trace Elem Res 201, 3311–3322 (2023). https://doi.org/10.1007/s12011-022-03438-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03438-6