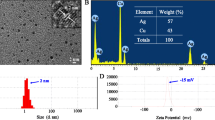
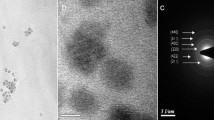
Abstract
Nanoparticles have garnered considerable scientific attention in recent years due to their diagnostic and therapeutic applications in cancer. The purpose of this study was to determine the effect of superparamagnetic iron oxide nanoparticles (Fe3O4 MNPs) on the induction of apoptosis in human colorectal adenocarcinoma cell line (HT-29) cells. The purpose of this study was to elucidate the mechanisms of apoptosis induced by Fe3O4 MNPs following MTT assay and to determine the optimal dose of 2.5 g/mL for inducing apoptosis in HT-29 cells. In HT-29 cells, Fe3O4 MNPs increased reactive oxygen species (ROS), calcium ion (Ca2+), and DNA damage. Additionally, the Fe3O4 MNPs significantly increased caspase 3 and 9 expression and decreased Bcl-2 expression at the protein and mRNA levels when compared to the control group (P = 0.0001). Fe3O4 MNPs also induced apoptosis in cancer cells by increasing the level of (ROS) and intracellular Ca2+, followed by an increase in caspase 3 and 9 expression and a decrease in Bcl-2 expression and direct DNA damage. Fe3O4 MNPs are an appropriate choice for colon cancer treatment based on their cell toxicity and induction of apoptosis in HT29 cells.




Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Fe3O4 MNPs:
-
Superparamagnetic iron oxide nanoparticles
- HT-29:
-
Human colorectal adenocarcinoma cell line (ATCC HTB-38)
- ROS:
-
Reactive oxygen species
- Bcl-2:
-
B cell lymphoma 2
- Bax:
-
BCL2-associated X
- PI:
-
Propidium iodide
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- BrdU:
-
Bromodeoxyuridine/5-bromo-2′-deoxyuridine
- NP:
-
Nanoparticle
- ELISA:
-
Enzyme-linked immunosorbent assay
References
Ranjbary AG, Mehrzad J, Dehghani H, Abdollahi A, Hosseinkhani S (2020) Variation in blood and colorectal epithelia’s key trace elements along with expression of mismatch repair proteins from localized and metastatic colorectal cancer patients. Biol Trace Elem Res 194(1):66–75. https://doi.org/10.1007/s12011-019-01749-9
Azimi M, Mehrzad J, Ahmadi A, Ahmadi E, Ghorbani Ranjbary A (2021) Apoptosis induced by Ziziphora tenuior essential oil in human colorectal cancer cells. BioMed Res Int 2021:9. https://doi.org/10.1155/2021/5522964
Siegel RL, Jakubowski CD, Fedewa SA, Davis A, Azad NS (2020) Colorectal cancer in the young: epidemiology, prevention, management. Am Soc Clin Oncol Educ Book 40:e75–e88. https://doi.org/10.1200/EDBK_279901
Arruebo M, Vilaboa N, Sáez-Gutierrez B, Lambea J, Tres A, Valladares M, González-Fernández Á (2011) Assessment of the evolution of cancer treatment therapies. Cancers 3(3):3279–3330. https://doi.org/10.3390/cancers3033279
Mokhtari RB, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, Yeger H (2017) Combination therapy in combating cancer. Oncotarget 8(23):38022. https://doi.org/10.18632/oncotarget.16723
Boateng F, Ngwa W (2020) Delivery of nanoparticle-based radiosensitizers for radiotherapy applications. Int J Mol Sci 21(1):273. https://doi.org/10.3390/ijms21010273
Zhao CY, Cheng R, Yang Z, Tian ZM (2018) Nanotechnology for cancer therapy based on chemotherapy. Molecules 23(4):826. https://doi.org/10.3390/molecules23040826
Powell CD, Atkinson AJ, Ma Y, Marcos-Hernandez M, Villagran D, Westerhoff P, Wong MS (2020) Magnetic nanoparticle recovery device (MagNERD) enables application of iron oxide nanoparticles for water treatment. J Nanopart Res 22(2):1–11. https://doi.org/10.1007/s11051-020-4770-4
Ganapathe LS, Mohamed MA, Mohamad Yunus R, Berhanuddin DD (2020) Magnetite (Fe3O4) nanoparticles in biomedical application: from synthesis to surface functionalisation. Magnetochemistry 6(4):68. https://doi.org/10.3390/magnetochemistry6040068
Namvar F, Rahman HS, Mohamad R et al (2014) Cytotoxic effect of magnetic iron oxide nanoparticles synthesized via seaweed aqueous extract. Int J Nanomed 9:2479. https://doi.org/10.2147/IJN.S59661
Kargar PG, Noorian M, Chamani E, Bagherzade G, Kiani Z (2021) Synthesis, characterization and cytotoxicity evaluation of a novel magnetic nanocomposite with iron oxide deposited on cellulose nanofibers with nickel (Fe3O4@ NFC@ ONSM-Ni). RSC Adv 11(28):17413–17430. https://doi.org/10.1039/D1RA01256H
Wu M, Huang S (2017) Magnetic nanoparticles in cancer diagnosis, drug delivery and treatment. Mol Clin Oncol 7(5):738–746. https://doi.org/10.3892/mco.2017.1399
Parodi A, Rudzinska M, Leporatti S, Anissimov Y, Zamyatnin AA Jr (2020) Smart nanotheranostics responsive to pathological stimuli. Front Bioeng Biotechnol 8:503. https://doi.org/10.3389/fbioe.2020.00503
Xinying L, Weidong G (2020) The emerging role of ultrasonic nanotechnology for diagnosing and treatment of diseases. Front Med 9:1–13. https://doi.org/10.3389/fmed.2022.814986
Thorat ND, Tofail SA, von Rechenberg B, Townley H, Brennan G, Silien C, Yadav HM, Steffen T, Bauer J (2019) Physically stimulated nanotheranostics for next generation cancer therapy: focus on magnetic and light stimulations. Appl Phys Rev 6(4):041306. https://doi.org/10.1063/1.5049467
Mohammed ET, Hashem KS, Abdelazem AZ, Foda FA (2020) Prospective protective effect of ellagic acid as a SIRT1 activator in iron oxide nanoparticle-induced renal damage in rats. Biol Trace Elem Res 198(1):177–88. https://doi.org/10.1007/s12011-020-02034-w
Bustamante-Torres M, Romero-Fierro D, Estrella-Nuñez J, Arcentales-Vera B, Chichande-Proaño E, Bucio E (2022) Polymeric composite of magnetite iron oxide nanoparticles and their application in biomedicine: a review. Polymers 14(4):752. https://doi.org/10.3390/polym14040752
Ghazanfari MR, Kashefi M, Shams SF, Jaafari MR (2016) Perspective of Fe3O4 nanoparticles role in biomedical applications. Biochem Res Int 2016:32. https://doi.org/10.1155/2016/7840161
Ganapathe LS, Mohamed MA, Mohamad Yunus R, Berhanuddin DD (2020) Magnetite (Fe3O4) nanoparticles in biomedical application: from synthesis to surface functionalisation. Magnetochemistry 6(4):68. https://doi.org/10.3390/magnetochemistry6040068
Christiansen MG, Howe CM, Bono DC, Perreault DJ, Anikeeva P (2017) Practical methods for generating alternating magnetic fields for biomedical research. Rev Sci Instrum 88(8):084301. https://doi.org/10.1063/1.4999358
Chen D, Tang Q, Li X, Zhou X, Zang J, Xue WQ, Xiang JY, Guo CQ (2012) Biocompatibility of magnetic Fe3O4 nanoparticles and their cytotoxic effect on MCF-7 cells. Int J Nanomed 7:4973. https://doi.org/10.2147/IJN.S35140
Wu M, Huang S (2017) Magnetic nanoparticles in cancer diagnosis, drug delivery and treatment. Mol Clin Oncol 7(5):738–746. https://doi.org/10.3892/mco.2017.1399
Sun C, Lee JSH, Zhang M (2008) Magnetic nanoparticles in MR imaging and drug delivery. Adv Drug Deliv Rev 60(11):1252–1265. https://doi.org/10.1016/j.addr.2008.03.018
Manke A, Wang L, Rojanasakul Y (2013) Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res Int 2013:15. https://doi.org/10.1155/2013/942916
Yu Z, Li Q, Wang J, Yu Y, Wang Y, Zhou Q, Li P (2020) Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Res Lett 15:1–4. https://doi.org/10.1186/s11671-020-03344-7
Zhang S, Wu S, Shen Y, Xiao Y, Gao L, Shi S (2020) Cytotoxicity studies of Fe3O4 nanoparticles in chicken macrophage cells. Royal Soc Open Sci 7(4):191561. https://doi.org/10.1098/rsos.191561
Huang YW, Cambre M, Lee HJ (2017) The toxicity of nanoparticles depends on multiple molecular and physicochemical mechanisms. Int J Mol Sci 18(12):2702. https://doi.org/10.3390/ijms18122702
Lujan H, Sayes CM (2017) Cytotoxicological pathways induced after nanoparticle exposure: studies of oxidative stress at the ‘nano-bio’ interface. Toxicol Res 6(5):580–94. https://doi.org/10.1039/C7TX00119C
Fu PP, Xia Q, Hwang HM, Ray PC, Yu H (2014) Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal 22(1):64–75. https://doi.org/10.1016/j.jfda.2014.01.005
Senapati S, Mahanta AK, Kumar S, Maiti P (2018) Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther 3(1):1–9. https://doi.org/10.1038/s41392-017-0004-3
Rashid H, Mansoor MA, Haider B, Nasir R, Abd Hamid SB, Abdulrahman A (2020) Synthesis and characterization of magnetite nano particles with high selectivity using in-situ precipitation method. Sep Sci Technol 55(6):1207–15. https://doi.org/10.1080/01496395.2019.1585876
Lodhia J, Mandarano G, Ferris NJ, Eu P, Cowell SF (2010) Development and use of iron oxide nanoparticles (Part 1): synthesis of iron oxide nanoparticles for MRI. Biomed Imaging Intervention J 6(2):e12. https://doi.org/10.2349/biij.6.2.e12
Hebling J, Bianchi L, Basso FG, Scheffel DL, Soares DG, Carrilho MR, Pashley DH, Tjäderhane L, de Souza Costa CA (2015) Cytotoxicity of dimethyl sulfoxide (DMSO) in direct contact with odontoblast-like cells. Dent Mater 31(4):399–405. https://doi.org/10.1016/j.dental.2015.01.007
Ho K, Yazan LS, Ismail N, Ismail M (2009) Apoptosis and cell cycle arrest of human colorectal cancer cell line HT-29 induced by vanillin. Cancer Epidemiol 33(2):155–60. https://doi.org/10.1016/j.canep.2009.06.003
Kuzmenka D, Sewohl C, König A, Flath T, Hahnel S, Schulze FP, Hacker MC, Schulz-Siegmund M (2020) Sustained calcium (II)-release to impart bioactivity in hybrid glass scaffolds for bone tissue engineering. Pharmaceutics 12(12):1192. https://doi.org/10.3390/pharmaceutics12121192
Zhang L, Chen H, Wang M, Song X, Ding F, Zhu J, Li X (2018) Effects of glabridin combined with 5-fluorouracil on the proliferation and apoptosis of gastric cancer cells. Oncol Lett 15(5):7037–45. https://doi.org/10.3892/ol.2018.8260
Gagnon M, ZihlerBerner A, Chervet N, Chassard C, Lacroix C (2013) Comparison of the Caco-2, HT-29 and the mucus-secreting HT29-MTX intestinal cell models to investigate Salmonella adhesion and invasion. J Microbiol Methods 94(3):274–9. https://doi.org/10.1016/j.mimet.2013.06.027
Soleymani J, Hasanzadeh M, Somi MH, Shadjou N, Jouyban A (2019) Highly sensitive and specific cytosensing of HT 29 colorectal cancer cells using folic acid functionalized-KCC-1 nanoparticles. Biosens Bioelectron 132:122–131. https://doi.org/10.1016/j.bios.2019.02.052
Tian L, Chen BA, Cheng J, Guo QL (2015) Effects of magnetic nanoparticles of Fe3O4 combinated with gambogic acid on apoptosis of SMMC-7721 cells. OncoTargets Ther 8:2285. https://doi.org/10.2147/OTT.S86494
Kojima K, Takahashi S, Saito S, Endo Y, Nittami T, Nozaki T, Sobti RC, Watanabe M (2018) Combined effects of Fe3O4 nanoparticles and chemotherapeutic agents on prostate cancer cells in vitro. Appl Sci 8(1):134. https://doi.org/10.3390/app8010134
Almukhlafi H, Ali D, Almutairi B, Yaseen KN, Alyami N, Almeer R, Alkahtani S, Alarifi S (2021) Role of Oxidative Stress in La2O3 Nanoparticle-Induced Cytotoxicity and Apoptosis in CHANG and HuH-7 Cells. Int J Nanomedicine 16:3487–3496. https://doi.org/10.2147/IJN.S302478
Jawaid P, Rehman MU, Zhao QL, Misawa M, Ishikawa K, Hori M, Shimizu T, Saitoh JI, Noguchi K, Kondo T (2020) Small size gold nanoparticles enhance apoptosis-induced by cold atmospheric plasma via depletion of intracellular GSH and modification of oxidative stress. Cell Death Discov 6(1):1–2. https://doi.org/10.1038/s41420-020-00314-x
Sato A, Itcho N, Ishiguro H, Okamoto D, Kobayashi N, Kawai K, Kasai H, Kurioka D, Uemura H, Kubota Y, Watanabe M (2013) Magnetic nanoparticles of Fe3O4 enhance docetaxel-induced prostate cancer cell death. Int J Nanomed 8:3151. https://doi.org/10.2147/IJN.S40766
Shen S, Liu Y, Huang P, Wang J (2009) In vitro cellular uptake and effects of Fe3O4 magnetic nanoparticles on HeLa cells. J Nanosci Nanotechnol 9(5):2866–71. https://doi.org/10.1166/jnn.2009.048
Mustafa T, Watanabe F, Monroe W, Mahmood M, Xu Y, Saeed LM, Karmakar A, Casciano D, All S, Biris AS (2011) Impact of gold nanoparticle concentration on their cellular uptake by MC3T3-E1 mouse osteoblastic cells as analyzed by transmission electron microscopy. J Nanomed Nanotechnol 2(6):1000118. https://doi.org/10.1017/S1431927611002297
Liang YQ, Chen BA, Wu WW, Gao F, Xia GH, Shao ZY, Cheng J, Ding JH, Gao C, Li GH, Chen WJ (2010) Effects of magnetic nanoparticle of Fe3O4 on apoptosis induced by Gambogic acid in U937 leukemia cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 18(1):67–73
Xie X, Zhang X, Chen J, Tang X, Wang M, Zhang L, Guo Z, Shen W (2019) Fe3O4-solamargine induces apoptosis and inhibits metastasis of pancreatic cancer cells. Int J Oncol 54(3):905–15. https://doi.org/10.3892/ijo.2018.4637
Kaplan A, Kutlu HM, Ciftci GA (2020) Fe3O4 nanopowders: genomic and apoptotic evaluations on A549 lung adenocarcinoma cell line. Nutr Cancer 72(4):708–21. https://doi.org/10.1080/01635581.2019.1643031
Chauhan A, Kumar R, Singh P, Jha SK, Kuanr BK (2020) RF hyperthermia by encapsulated Fe3O4 nanoparticles induces cancer cell death via time-dependent caspase-3 activation. Nanomedicine 15(04):355–79. https://doi.org/10.2217/nnm-2019-0187
Ozaki T, Nakagawara A (2011) Role of p53 in cell death and human cancers. Cancers 3(1):994–1013. https://doi.org/10.3390/cancers3010994
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516. https://doi.org/10.1080/01926230701320337
Ivanova D, Zhelev Z, Aoki I, Bakalova R, Higashi T (2016) Overproduction of reactive oxygen species—obligatory or not for induction of apoptosis by anticancer drugs. Chin J Cancer Res 28(4):383. https://doi.org/10.21147/j.issn.1000-9604.2016.04.01
Ott M, Gogvadze V, Orrenius S, Zhivotovsky B (2007) Mitochondria, oxidative stress and cell death. Apoptosis 12(5):913–22. https://doi.org/10.1007/s10495-007-0756-2
Kasai S, Shimizu S, Tatara Y, Mimura J, Itoh K (2020) Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules 10(2):320. https://doi.org/10.3390/biom10020320
Gurunathan S, Kang MH, Kim JH (2018) Combination Effect of Silver Nanoparticles and Histone Deacetylases Inhibitor in Human Alveolar Basal Epithelial Cells. Molecules 23(8):2046. https://doi.org/10.3390/molecules23082046
Redza-Dutordoir M (1863) Averill-Bates DA (2016) Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta (BBA)- Mol Cell Res 12:2977–92. https://doi.org/10.1016/j.bbamcr.2016.09.012
Giacomello M, Drago I, Pizzo P, Pozzan T (2007) Mitochondrial Ca2+ as a key regulator of cell life and death. Cell Death Differ 14(7):1267–74. https://doi.org/10.1038/sj.cdd.4402147
Salminen A, Kaarniranta K, Kauppinen A (2013) Crosstalk between oxidative stress and SIRT1: impact on the aging process. IJMS 14(2):3834–3859. https://doi.org/10.3390/ijms14023834
He X, Maimaiti M, Jiao Y et al (2018) Sinomenine induces G1-phase cell cycle arrest and apoptosis in malignant glioma cells via downregulation of sirtuin 1 and induction of p53 acetylation. Technol Cancer Res Treat 17:153303461877030. https://doi.org/10.1177/1533034618770305
Singh CK, Chhabra G, Ndiaye MA, Garcia-Peterson LM, Mack NJ, Ahmad N (2018) The role of sirtuins in antioxidant and redox signaling. Antioxid Redox Signal 28(8):643–661. https://doi.org/10.1089/ars.2017.7290
Wang Z (2021) Regulation of cell cycle progression by growth factor-induced cell signaling. Cells 10(12):3327. https://doi.org/10.3390/cells10123327
Li Y, Qin Y, Yang C, Zhang H, Li Y, Wu B, Huang J, Zhou X, Huang B, Yang K, Wu G (2017) Cardamonin induces ROS-mediated G2/M phase arrest and apoptosis through inhibition of NF-κB pathway in nasopharyngeal carcinoma. Cell Death Dis 8(8):e3024. https://doi.org/10.1038/cddis.2017.407
Aubrey BJ, Kelly GL, Janic A, Herold MJ, Strasser A (2018) How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ 25(1):104–13. https://doi.org/10.1038/cdd.2017.169
Cai X, Wang C, Chen B, Hua W, Shen F, Yu L, He Z, Shi Y, Chen Y, Xia G, Cheng J (2014) Antitumor efficacy of DMSA modified Fe3O4 magnetic nanoparticles combined with arsenic trioxide and adriamycin in Raji cells. J Biomed Nanotechnol 10(2):251–61. https://doi.org/10.1166/jbn.2014.1787
Feno S, Butera G, VecellioReane D, Rizzuto R, Raffaello A (2019) Crosstalk between calcium and ROS in pathophysiological conditions. Oxidative Med Cell Longevity 24(2):19. https://doi.org/10.1155/2019/9324018
Zhu L, Han MB, Gao Y, Wang H, Dai L, Wen Y, Na LX (2015) Curcumin triggers apoptosis via upregulation of Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes. Mol Med Rep 12(1):1151–6. https://doi.org/10.3892/mmr.2015
Alarifi S, Ali H, SaadAlkahtani MS (2017) Regulation of apoptosis through Bcl-2/Bax proteins expression and DNA damage by nano-sized gadolinium oxide. Int J Nanomed 12:4541. https://doi.org/10.2147/IJN.S139326
Yue J, López JM (2020) Understanding MAPK signaling pathways in apoptosis. Int J Mol Sci 21(7):2346. https://doi.org/10.3390/ijms21072346
Guerrero AD, Schmitz I, Chen M, Wang J (2012) Promotion of caspase activation by caspase-9-mediated feedback amplification of mitochondrial damage. J Clin Cell Immunol 3(3):1000126. https://doi.org/10.4172/2155-9899.1000126
Naseri MH, Mahdavi M, Davoodi J, Tackallou SH, Goudarzvand M, Neishabouri SH (2015) Up regulation of Bax and down regulation of Bcl2 during 3-NC mediated apoptosis in human cancer cells. Cancer Cell Int 15:55. https://doi.org/10.1186/s12935-015-0204-2
Raisova M, Hossini AM, Eberle J, Riebeling C, Wieder T, Sturm I, Daniel PT, Orfanos CE, Geilen CC (2001) The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J Invest Dermatol 117(2):333–40. https://doi.org/10.1046/j.0022-202x.2001.01409.x
Kulsoom B, Shamsi TS, Afsar NA, Memon Z, Ahmed N, Hasnain SN (2018) Bax, Bcl-2, and Bax/Bcl-2 as prognostic markers in acute myeloid leukemia: are we ready for Bcl-2-directed therapy? Cancer Manag Res 10:403–416. https://doi.org/10.2147/CMAR.S154608
Zhang XY, Guo XZ, Wu SX (2021) Up-regulation of Bax/BCL-2 ratio by 2-methoxyestradiol induces apoptosis in lymphoma Raji cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 29(2):489–493. https://doi.org/10.19746/j.cnki.issn.1009-2137.2021
Acknowledgements
The authors thank Dr. Reza Rastegar University of Tehran Laboratory and Ferdowsi University of Mashhad Central Laboratory for their cooperation.
Author information
Authors and Affiliations
Contributions
Mohammadreza Azimi, Jalil Mehrzad, Golnaz Karbalaei Saleh and Ali Ghorbani Ranjbary performed the experiments, conceived and designed the study, and wrote, analyzed, funded, and critically revised the manuscript. Fatemeh karimian and Javad zohdi also actively helped in the experiments and participated in study design, study implementation, and manuscript revision.
Corresponding author
Ethics declarations
Ethics Approval
This study has been approved by the Ethics Committee of the Tehran University of Tehran, Tehran, Iran.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ranjbary, A.G., Saleh, G.K., Azimi, M. et al. Superparamagnetic Iron Oxide Nanoparticles Induce Apoptosis in HT-29 Cells by Stimulating Oxidative Stress and Damaging DNA. Biol Trace Elem Res 201, 1163–1173 (2023). https://doi.org/10.1007/s12011-022-03229-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03229-z