Abstract
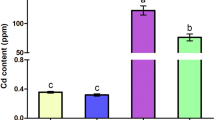
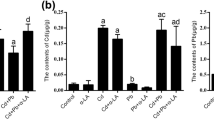
Excess molybdenum (Mo) and cadmium (Cd) are harmful to animals, but the neurotoxic mechanism co-induced by Mo and Cd is unclear. To estimate the effects of Mo and Cd co-exposure on pyroptosis by nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated antioxidant defense response in duck brains, 40 healthy 7-day-old ducks were randomly assigned to 4 groups and fed diet supplemented with Mo or/and Cd for 16 weeks, respectively. Results showed that Mo or/and Cd markedly increased Mo and Cd contents; decreased iron (Fe), copper (Cu), zinc (Zn), and selenium (Se) contents, elevated malondialdehyde (MDA) content; and decreased total-antioxidant capacity (T-AOC), total-superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) activities accompanied by pathological damage in brain. Additionally, Mo or/and Cd inhibited Nrf2 pathway via decreasing Nrf2, CAT, SOD1, glutathione S-transferase (GST), hemeoxygenase-1 (HO-1), NAD (P) H:quinone oxidoreductase 1 (NQO1), glutamate-cysteine ligase catalytic subunit (GCLC), and modifier subunit (GCLM) mRNA levels and Nrf2 protein level, which induced pyroptosis through upregulating nucleotide oligomerization domain-like receptor protein-3 (NLRP3), apoptosis-associated speck-like protein (ASC), gasdermin A (GSDMA), gasdermin E (GSDME), interleukin-1β (IL-1β), interleukin-18 (IL-18), Caspase-1, NIMA-related kinase 7 (NEK7) mRNA levels and NLRP3, Caspase-1 p20, gasdermin D (GSDMD), ASC protein levels and IL-1β, and IL-18 contents. Besides, the changes of these indicators were most apparent in the Mo and Cd co-treated group. Collectively, the results certificated that Mo and Cd might synergistically induce pyroptosis via inhibiting Nrf2-mediated antioxidant defense response in duck brains, whose mechanism is closely related to Mo and Cd accumulation.







Similar content being viewed by others
Data availability
All data generated or analyzed in this study are included in this article.
References
Mendel RR (2009) Cell biology of molybdenum. BioFactors 35(5):429–434. https://doi.org/10.1002/biof.55
Novotny JA, Peterson CA (2018) Molybdenum Advances in nutrition 9(3):272–273. https://doi.org/10.1093/advances/nmx001
Wang HW, Zhou BH, Zhang S, Guo HW, Zhang JL, Zhao J, Tian EJ (2016) Reproductive toxicity in male mice after exposure to high molybdenum and low copper concentrations. Toxicol Ind Health 32(9):1598–1606. https://doi.org/10.1177/0748233715569269
Jarrell WM, Page AL, Elseewi AA (1980) Molybdenum in the environment. Residue Rev 74:1–43. https://doi.org/10.1007/978-1-4612-6096-7_1
Timofeev I, Kosheleva N, Kasimov N (2018) Contamination of soils by potentially toxic elements in the impact zone of tungsten-molybdenum ore mine in the Baikal region: a survey and risk assessment. Sci Total Environ 642:63–76. https://doi.org/10.1016/j.scitotenv.2018.06.042
Wang X, Brunetti G, Tian W, Owens G, Qu Y, Jin C, Lombi E (2021) Effect of soil amendments on molybdenum availability in mine affected agricultural soils. Environ Pollut 269:116132. https://doi.org/10.1016/j.envpol.2020.116132
Xu S, Hu C, Tan Q, Qin S, Sun X (2018) Subcellular distribution of molybdenum, ultrastructural and antioxidative responses in soybean seedlings under excess molybdenum stress. Plant phys biochem PPB 123:75–80. https://doi.org/10.1016/j.plaphy.2017.11.023
Shi L, Cao H, Luo J, Liu P, Wang T, Hu G, Zhang C (2017) Effects of molybdenum and cadmium on the oxidative damage and kidney apoptosis in duck. Ecotoxicol Environ Saf 145:24–31. https://doi.org/10.1016/j.ecoenv.2017.07.006
Cao H, Gao F, Xia B, Zhang M, Liao Y, Yang Z, Hu G, Zhang C (2016) Alterations in trace element levels and mRNA expression of Hsps and inflammatory cytokines in livers of duck exposed to molybdenum or/and cadmium. Ecotoxicol Environ Saf 125:93–101. https://doi.org/10.1016/j.ecoenv.2015.12.003
Yang P, Ke S, Tu L, Wang Y, Ye S, Kou S, Ren L (2020) Regulation of autophagy orchestrates pyroptotic cell death in molybdenum disulfide quantum dot-induced microglial toxicity. ACS Biomater Sci Eng 6(3):1764–1775. https://doi.org/10.1021/acsbiomaterials.9b01932
Loganathan P, Hedley MJ, Grace ND (2008) Pasture soils contaminated with fertilizer-derived cadmium and fluorine: livestock effects. Rev Environ Contam Toxicol 192:29–66. https://doi.org/10.1007/978-0-387-71724-1_2
Wang B, Du Y (2013) Cadmium and its neurotoxic effects. Oxid Med Cell Longev 2013:898034. https://doi.org/10.1155/2013/898034
Bandara JM, Wijewardena HV, Bandara YM, Jayasooriya RG, Rajapaksha H (2011) Pollution of River Mahaweli and farmlands under irrigation by cadmium from agricultural inputs leading to a chronic renal failure epidemic among farmers in NCP. Sri Lanka Environmental geochemistry and health 33(5):439–453. https://doi.org/10.1007/s10653-010-9344-4
Zhang X, Chen D, Zhong T, Zhang X, Cheng M, Li X (2015) Assessment of cadmium (Cd) concentration in arable soil in China. Environ Sci Pollut Res Int 22(7):4932–4941. https://doi.org/10.1007/s11356-014-3892-6
Liu ZP (2003) Lead poisoning combined with cadmium in sheep and horses in the vicinity of non-ferrous metal smelters. Sci Total Environ 309(1–3):117–126. https://doi.org/10.1016/S0048-9697(03)00011-1
Liu S, Fu Y, Shi M, Wang H, Guo J (2021) Pollution level and risk assessment of lead, cadmium, mercury, and arsenic in edible mushrooms from Jilin Province. China J food sci 86(8):3374–3383. https://doi.org/10.1111/1750-3841.15849
Chen X, Bi M, Yang J, Cai J, Zhang H, Zhu Y, Zheng Y, Liu Q, Shi G, Zhang Z (2022) Cadmium exposure triggers oxidative stress, necroptosis, Th1/Th2 imbalance and promotes inflammation through the TNF-α/NF-κB pathway in swine small intestine. J Hazard Mater 421:126704. https://doi.org/10.1016/j.jhazmat.2021.126704
Gong ZG, Zhao Y, Wang ZY, Fan RF, Liu ZP, Wang L (2022) Epigenetic regulator BRD4 is involved in cadmium-induced acute kidney injury via contributing to lysosomal dysfunction, autophagy blockade and oxidative stress. J Hazard Mater 423(Pt A):127110. https://doi.org/10.1016/j.jhazmat.2021.127110
Khan A, Ikram M, Muhammad T, Park J, Kim MO (2019) Caffeine modulates cadmium-induced oxidative stress, neuroinflammation, and cognitive impairments by regulating Nrf-2/HO-1 in vivo and in vitro. J Clin Med 8(5):680. https://doi.org/10.3390/jcm8050680
Rinaldi M, Micali A, Marini H, Adamo EB, Puzzolo D, Pisani A, Trichilo V, Altavilla D, Squadrito F, Minutoli L (2017) Cadmium, organ toxicity and therapeutic approaches: a review on brain, kidney and testis damage. Curr Med Chem 24(35):3879–3893. https://doi.org/10.2174/0929867324666170801101448
Forcella M, Lau P, Oldani M, Melchioretto P, Bogni A, Gribaldo L, Fusi P, Urani C (2020) Neuronal specific and non-specific responses to cadmium possibly involved in neurodegeneration: a toxicogenomics study in a human neuronal cell model. Neurotoxicology 76:162–173. https://doi.org/10.1016/j.neuro.2019.11.002
Amuno S, Shekh K, Kodzhahinchev V, Niyogi S (2020) Neuropathological changes in wild muskrats (Ondatra zibethicus) and red squirrels (Tamiasciurus hudsonicus) breeding in arsenic endemic areas of Yellowknife, Northwest Territories (Canada): arsenic and cadmium accumulation in the brain and biomarkers of oxidative stress. Sci Total Environ 704:135426. https://doi.org/10.1016/j.scitotenv.2019.135426
Hybertson BM, Gao B, Bose SK, McCord JM (2011) Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med 32(4–6):234–246. https://doi.org/10.1016/j.mam.2011.10.006
Liao J, Yang F, Chen H, Yu W, Han Q, Li Y, Hu L, Guo J, Pan J, Liang Z, Tang Z (2019) Effects of copper on oxidative stress and autophagy in hypothalamus of broilers. Ecotoxicol Environ Saf 185:109710. https://doi.org/10.1016/j.ecoenv.2019.109710
Jiang X, Xing X, Zhang Y, Zhang C, Wu Y, Chen Y, Meng R, Jia H, Cheng Y, Zhang Y, Su J (2021) Lead exposure activates the Nrf2/Keap1 pathway, aggravates oxidative stress, and induces reproductive damage in female mice. Ecotoxicol Environ Saf 207:111231. https://doi.org/10.1016/j.ecoenv.2020.111231
Nazimabashir M, V, & Miltonprabu, S (2015) Cadmium induced cardiac oxidative stress in rats and its attenuation by GSP through the activation of Nrf2 signaling pathway. Chem Biol Interact 242:179–193. https://doi.org/10.1016/j.cbi.2015.10.005
Dai Z, Cheng J, Bao L, Zhu X, Li H, Chen X, Zhang Y, Zhang J, Chu W, Pan Y, Huang H (2020) Exposure to waterborne cadmium induce oxidative stress, autophagy and mitochondrial dysfunction in the liver of Procypris merus. Ecotoxicol Environ Saf 204:111051. https://doi.org/10.1016/j.ecoenv.2020.111051
Diao C, Chen Z, Qiu T, Liu H, Yang Y, Liu X, Wu J, Wang L (2019) Inhibition of PRMT5 attenuates oxidative stress-induced pyroptosis via activation of the Nrf2/HO-1 signal pathway in a mouse model of renal ischemia-reperfusion injury. Oxid Med Cell Longev 2019:2345658. https://doi.org/10.1155/2019/2345658
Ding R, Ou W, Chen C, Liu Y, Li H, Zhang X, Chai H, Ding X, Wang Q (2020) Endoplasmic reticulum stress and oxidative stress contribute to neuronal pyroptosis caused by cerebral venous sinus thrombosis in rats: involvement of TXNIP/peroxynitrite-NLRP3 inflammasome activation. Neurochem Int 141:104856. https://doi.org/10.1016/j.neuint.2020.104856
VandeWalle L, Lamkanfi M (2016) Pyroptosis. Current biol CB 26(13):R568–R572. https://doi.org/10.1016/j.cub.2016.02.019
Rubartelli A (2012) Redox control of NLRP3 inflammasome activation in health and disease. J Leukoc Biol 92(5):951–958. https://doi.org/10.1189/jlb.0512265
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526(7575):660–665. https://doi.org/10.1038/nature15514
Rheinheimer, J., de Souza, B. M., Cardoso, N. S., Bauer, A. C., & Crispim, D. (2017). Current role of the NLRP3 inflammasome on obesity and insulin resistance: A systematic review. Metabolism: clinical and experimental, 74, 1–9. https://doi.org/10.1016/j.metabol.2017.06.002
Wang L, Li K, Lin X, Yao Z, Wang S, Xiong X, Ning Z, Wang J, Xu X, Jiang Y, Liu D, Chen Y, Zhang D, Zhang H (2019) Metformin induces human esophageal carcinoma cell pyroptosis by targeting the miR-497/PELP1 axis. Cancer Lett 450:22–31. https://doi.org/10.1016/j.canlet.2019.02.014
Zhang C, Hu Z, Hu R, Pi S, Wei Z, Wang C, Yang F, Xing C, Nie G, Hu G (2021) New insights into crosstalk between pyroptosis and autophagy co-induced by molybdenum and cadmium in duck renal tubular epithelial cells. J Hazard Mater 416:126138. https://doi.org/10.1016/j.jhazmat.2021.126138
Miao Z, Miao Z, Shi X, Wu H, Yao Y, Xu S (2022) The antagonistic effect of selenium on lead-induced apoptosis and necroptosis via P38/JNK/ERK pathway in chicken kidney. Ecotoxicol Environ Saf 231:113176. https://doi.org/10.1016/j.ecoenv.2022.113176
Zhang C, Lin T, Nie G, Hu R, Pi S, Wei Z, Wang C, Li G, Hu G (2021) In vivo assessment of molybdenum and cadmium co-induce nephrotoxicity via causing calcium homeostasis disorder and autophagy in ducks (Anas platyrhyncha). Ecotoxicol Environ Saf 230:113099. https://doi.org/10.1016/j.ecoenv.2021.113099
Wei Z, Nie G, Yang F, Pi S, Wang C, Cao H, Guo X, Liu P, Li G, Hu G, Zhang C (2020) Inhibition of ROS/NLRP3/Caspase-1 mediated pyroptosis attenuates cadmium-induced apoptosis in duck renal tubular epithelial cells. Environ Pollut 273:115919. https://doi.org/10.1016/j.envpol.2020.115919
Liu G, Wang ZK, Wang ZY, Yang DB, Liu ZP, Wang L (2016) Mitochondrial permeability transition and its regulatory components are implicated in apoptosis of primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol 90(5):1193–1209. https://doi.org/10.1007/s00204-015-1547-0
Liu Q, Du P, Zhu Y, Zhang X, Cai J, Zhang Z (2022) Thioredoxin reductase 3 suppression promotes colitis and carcinogenesis via activating pyroptosis and necrosis. Cellular and molecular life sciences : CMLS 79(2):106. https://doi.org/10.1007/s00018-022-04155-y
Paithankar JG, Saini S, Dwivedi S, Sharma A, Chowdhuri DK (2021) Heavy metal associated health hazards: an interplay of oxidative stress and signal transduction. Chemosphere 262:128350. https://doi.org/10.1016/j.chemosphere.2020.128350
Liao Y, Cao H, Xia B, Xiao Q, Liu P, Hu G, Zhang C (2017) Changes in trace element contents and morphology in bones of duck exposed to molybdenum or/and cadmium. Biol Trace Elem Res 175(2):449–457. https://doi.org/10.1007/s12011-016-0778-0
Shao JJ, Yao HD, Zhang ZW, Li S, Xu SW (2012) The disruption of mitochondrial metabolism and ion homeostasis in chicken hearts exposed to manganese. Toxicol Lett 214(2):99–108. https://doi.org/10.1016/j.toxlet.2012.08.011
Diyabalanage S, Dangolla A, Mallawa C, Rajapakse S, Chandrajith R (2020) Bioavailability of selenium (Se) in cattle population in Sri Lanka based on qualitative determination of glutathione peroxidase (GSH-Px) activities. Environ Geochem Health 42(2):617–624. https://doi.org/10.1007/s10653-019-00395-3
Jomova K, Valko M (2011) Advances in metal-induced oxidative stress and human disease. Toxicology 283(2–3):65–87. https://doi.org/10.1016/j.tox.2011.03.001
Li Y, Shen X, Liu F, Luo L, Wang Y (2021) Molybdenum fertilization improved antioxidant capacity of grazing Nanjiang brown goat on Copper-contaminated pasture. Biol Trace Elem Res. https://doi.org/10.1007/s12011-021-02735-w
Ge J, Zhang C, Sun YC, Zhang Q, Lv MW, Guo K, Li JL (2019) Cadmium exposure triggers mitochondrial dysfunction and oxidative stress in chicken (Gallus gallus) kidney via mitochondrial UPR inhibition and Nrf2-mediated antioxidant defense activation. Sci Total Environ 689:1160–1171. https://doi.org/10.1016/j.scitotenv.2019.06.405
Tang KK, Li HQ, Qu KC, Fan RF (2019) Selenium alleviates cadmium-induced inflammation and meat quality degradation via antioxidant and anti-inflammation in chicken breast muscles. Environ Sci Pollut Res Int 26(23):23453–23459. https://doi.org/10.1007/s11356-019-05675-0
Wang Y, Chen H, Chang W, Chen R, Xu S, Tao D (2020) Protective effects of selenium yeast against cadmium-induced necroptosis via inhibition of oxidative stress and MAPK pathway in chicken liver. Ecotoxicol Environ Saf 206:111329. https://doi.org/10.1016/j.ecoenv.2020.111329
Koto KS, Lescault P, Brard L, Kim K, Singh RK, Bond J, Illenye S, Slavik MA, Ashikaga T, SaulnierSholler GL (2011) Antitumor activity of nifurtimox is enhanced with tetrathiomolybdate in medulloblastoma. Int J Oncol 38(5):1329–1341. https://doi.org/10.3892/ijo.2011.971
Helaly AM, Mokhtar N, Firgany A, Hazem NM, El Morsi E, Ghorab D (2018) Molybdenum bupropion combined neurotoxicity in rats. Regulatory toxicology and pharmacology : RTP 98:224–230. https://doi.org/10.1016/j.yrtph.2018.08.001
Das S, Dewanjee S, Dua TK, Joardar S, Chakraborty P, Bhowmick S, Saha A, Bhattacharjee S, De Feo V (2019) Carnosic acid attenuates Cadmium induced nephrotoxicity by inhibiting oxidative stress, promoting Nrf2/HO-1 signalling and impairing TGF-β1/Smad/Collagen IV signalling. Molecules 24(22):4176. https://doi.org/10.3390/molecules24224176
Liu C, Zhu Y, Lu Z, Guo W, Tumen B, He Y, Chen C, Hu S, Xu K, Wang Y, Li L, Li S (2019) Cadmium induces acute liver injury by inhibiting Nrf2 and the role of NF-κB, NLRP3, and MAPKs signaling pathway. Int J Environ Res Public Health 17(1):138. https://doi.org/10.3390/ijerph17010138
Cai J, Guan H, Jiao X, Yang J, Chen X, Zhang H, Zheng Y, Zhu Y, Liu Q, Zhang Z (2021) NLRP3 inflammasome mediated pyroptosis is involved in cadmium exposure-induced neuroinflammation through the IL-1β/IkB-α-NF-κB-NLRP3 feedback loop in swine. Toxicology 453:152720. https://doi.org/10.1016/j.tox.2021.152720
Zhang Y, Liu Q, Yin H, Li S (2020) Cadmium exposure induces pyroptosis of lymphocytes in carp pronephros and spleens by activating NLRP3. Ecotoxicol Environ Saf 202:110903. https://doi.org/10.1016/j.ecoenv.2020.110903
Zhang C, Lin T, Nie G, Hu R, Pi S, Wei Z, Wang C, Xing C, Hu G (2021) Cadmium and molybdenum co-induce pyroptosis via ROS/PTEN/PI3K/AKT axis in duck renal tubular epithelial cells. Environ Pollut 272:116403. https://doi.org/10.1016/j.envpol.2020.116403
Zhao Y, Du ZH, Talukder M, Lin J, Li XN, Zhang C, Li JL (2018) Crosstalk between unfolded protein response and Nrf2-mediated antioxidant defense in Di-(2-ethylhexyl) phthalate-induced renal injury in quail (Coturnix japonica). Environ Pollut 242(Pt B):1871–1879. https://doi.org/10.1016/j.envpol.2018.07.080
Funding
The work was supported by the National Natural Science Foundation of China (No. 31960722).
Author information
Authors and Affiliations
Contributions
Zhisheng Hu: conceptualization, software, formal analysis, writing-original draft, data curation, visualization, writing-review and editing, validation. Gaohui Nie: methodology, visualization, resources. Junrong Luo: data curation, validation, formal analysis. Ruiming Hu: data curation, validation, formal analysis. Chenghong Xing: methodology, formal analysis. Guoliang Hu: resources, validation. Caiying Zhang: conceptualization, project administration, writing-review and editing, funding acquisition.
Corresponding author
Ethics declarations
Consent for publication
All authors have read the manuscript and agreed to submit it in its current form for consideration for publication in the Journal.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, Z., Nie, G., Luo, J. et al. Molybdenum and Cadmium Co-induce Pyroptosis via Inhibiting Nrf2-Mediated Antioxidant Defense Response in the Brain of Ducks. Biol Trace Elem Res 201, 874–887 (2023). https://doi.org/10.1007/s12011-022-03170-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03170-1