Abstract
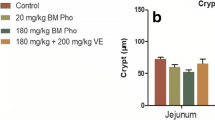
The aim of this study was to investigate the alleviating effect of methionine (Met) on intestinal injury induced by nickel. The mice were divided into six groups: Met-deficient + nickel group (MDN), Met-deficient group (MD), Met + nickel group (MN), high-dose Met + nickel group (HMN), high-dose Met group (HM), and blank control group (BC). Histopathological techniques, Alcian blue-periodic acid Schiff (AB-PAS) staining, enzyme-linked immunosorbent assay (ELISA), and real-time PCR were used to study the changes of intestinal development, the number of goblet cells, and the intercellular junction. The results showed that Met can inhibit the intestinal villus length and crypt depth decreases induced by nickel and increase the index villus length and crypt depth (V/C), the number of goblet cells, and the content of diamine oxidase (DAO) and decrease the content of fatty acid binding protein2 (FABP2) and endotoxin (ET) of the intestinal mucosa damage parameters, and the mRNA expression of intercellular junction (occludin, ZO-1, claudin-1) was damaged. It is suggested that Met could help inhibit the toxic effect of nickel on the intestinal development and intercellular connection.







Similar content being viewed by others
References
Lu CY, Lu XG, Zou X, Cheng H, Xu Q (2015) Current situation and utilization technology of nickel ore in China. Chinese J Nature 37(4):269–277. https://doi.org/10.3969/j.issn.0253-9608.2015.04.004
Liu G, Sun L, Pan A, Zhu M, Li Z, Wang Z, Liu X, Ye X, Li H, Zheng H (2015) Nickel exposure is associated with the prevalence of type 2 diabetes in Chinese adults. Int J Epidemiol 44(1):240–248. https://doi.org/10.1093/ije/dyu200
Akinwumi KA, Jubril AJ, Olaniyan OO, Umar YY (2020) Ethanol extract of nigella sativa has antioxidant and ameliorative effect against nickel chloride-induced hepato-renal injury in rats. Clin Phytoscience 6(1):64–76. https://doi.org/10.1186/s40816-020-00205-9
IARC (2012) A review of human carcinogens, part C: arsenic, metals, fibres and dust. In: IARC monographs on the evaluation of carcinogenic risks to humans, vol. 100C. Lyon: World Health Organization; p. 169–211
Akinci IE, Ongel O (2011) Amelioration of nickel toxicity by humic acid on bean (phaseolus vulgaris) seedling growth. Ekoloji 20(79):29–37. https://doi.org/10.5053/ekoloji.2011.794
Idrees M, Naeem M, Aftab T, Khan MMA, Moinuddin (2013) Salicylic acid restrains nickel toxicity, improves antioxidant defence system and enhances the production of anticancer alkaloids in Catharanthus roseus (L.). J Hazard Mater 252-253:367-374. https://doi.org/10.1016/j.jhazmat.2013.03.005
Pompeu GB, Gratão PL, Vitorello VA, Azevedo RA (2008) Antioxidant isoenzyme responses to nickel-induced stress in tobacco cell suspension culture resposta de isoenzimas antioxidantes ao estresse induzido por níquel em cultura de células em suspensão de fumo. Scientia Agricola 65(5):548–552. https://doi.org/10.1590/S0103-90162008000500015
Nielsen FH, Yokoi K, Uthus E (2000) Dietary nickel alters the response of rats to deficient dietary pyridoxine, and to high dietary homocystine or methionine) [abstract]. J Fed Am Soc Exp Biol 14:A539
Čolović MB, Vasić VM, Djuri DM, Krstić DZ (2017) Sulphur-containing amino acids: protective role against free radicals and heavy metals. Curr Med Chem 25(3):1–12. https://doi.org/10.2174/0929867324666170609075434
Salmon AB, Kim G, Liu C, Wren JD, Georgescu C, Richardson A, Levine RL (2016) Effects of transgenic methionine sulfoxide reductase a (msra) expression on lifespan and age-dependent changes in metabolic function in mice. Redox Biol 10:251–256. https://doi.org/10.1016/j.redox.2016.10.012
Demerchi SA, Mcfarlane JR, King N, Moens P (2020) Effect of l-methionine feeding on serum homocysteine and glutathione levels in male and female Wistar rats. Adv Biochem 8(1):21–25. https://doi.org/10.11648/j.ab.20200801.14
Tamanna N, Kroeker K, Braun K, Banh S, Treberg JR (2019) The effect of short-term methionine restriction on glutathione synthetic capacity and antioxidant responses at the whole tissue and mitochondrial level in the rat liver-sciencedirect. Exp Gerontol 127:110712–110712. https://doi.org/10.1016/j.exger.2019.110712
Sanchez-Roman I, Gomez A, Gomez J, Suarez H, Sanchez C, Naudi A, Ayala V, Portero-Otin M, Lopez-Torres M, Pamplona R, Barja G (2011) Forty percent methionine restriction lowers DNA methylation, complex I ROS generation, and oxidative damage to mtDNA and mitochondrial proteins in rat heart. J Bioenerg Biomembr 43(6):699–708. https://doi.org/10.1007/s10863-011-9389-9
Varga Z, Karpati I, Biro S, Matyus J, Ujhelyi L, Papp L, Posta J, Paragh G, Balla J (2006) Nickel affects methionine cycle by a concentration dependent manner and resulted in decreased homocysteine and S-adenosylhomocysteine concentration in vitro and in vivo[C]//Nephrology Dialysis Transplantation. Great Clarendon St, Oxford Ox2 6dp, England: Oxford Univ Press, 21: 189–189.
Uthus EO, Poellot RA (1997) Dietary nickel and folic acid interact to affect folate and methionine metabolism in the rat. Biol Trace Elem Res 58(1–2):25–33. https://doi.org/10.1007/BF02910663
Ding X, Hua Y, Chen Y, Zhang C, Kong X (2015) Heavy metal complexation of thiol-containing peptides from soy glycinin hydrolysates. Int J Mol Sci 16(4):8040–8058. https://doi.org/10.3390/ijms16048040
Feldman AT, Wolfe D (2014) Tissue processing and hematoxylin and eosin staining. Methods Mol Biol 1180:31–43. https://doi.org/10.1007/978-1-4939-1050-2_3
Yamabayashi S (1987) Periodic acid - schiff - alcian blue: a method for the differential staining of glycoproteins. Histochem J 19(10–11):565–571. https://doi.org/10.1007/BF01687364
Wu B, Cui H, Peng X, Fang J, Zuo Z, Deng J, Huang J (2014) Dietary nickel chloride induces oxidative stress, apoptosis and alters Bax/Bcl-2 and caspase-3 mRNA expression in the cecal tonsil of broilers. Food Chem Toxicol 63:18–29. https://doi.org/10.1016/j.fct.2013.10.033
Hamada K, Kakigawa N, Sekine S, Shitara Y, Horie T (2013) Disruption of zo-1/claudin-4 interaction in relation to inflammatory responses in methotrexate-induced intestinal mucositis. Cancer Chemother Pharmacol 72(4):757–765. https://doi.org/10.1007/s00280-013-2238-2
Wu BY, Cui HM, Peng X, Jing F, Zuo Z, Deng J, Huang J (2013) Dietary nickel chloride induces oxidative intestinal damage in broilers. Int J Env Res Pub He 10(6):2109–2119. https://doi.org/10.3390/ijerph10062109
Das KK, Das SN, Dhundasi SA (2008) Nickel, its adverse health effects & oxidative stress. Indian J Med Res 128(4):412–425
Collier-Hyams LS, Neish AS (2005) Intestinal epithelial barrier and mucosal immunity. Cell Mol Life Sci 62(12):1339–1348. https://doi.org/10.1007/s00018-005-5038-y
Yang CM, Han QJ, Wang KL, Xu YL, Lan JH, Cao GT (2019) Astragalus and ginseng polysaccharides improve developmental, intestinal morphological, and immune functional characters of weaned piglets. Front Physiol 10:418–429. https://doi.org/10.3389/fphys.2019.00418
He X, Lin Y, Lian S, Sun D, Wu R (2020) Selenium deficiency in chickens induces intestinal mucosal injury by affecting the mucosa morphology, siga secretion, and gsh-px activity. Biol Trace Elem Res 197(2):660–666. https://doi.org/10.1007/s12011-019-02017-6
Shoveller AK, Stoll B, Ball RO, Burrin DG (2005) Nutritional and functional importance of intestinal sulfur amino acid metabolism. J Nutr 135(7):1609–1612. https://doi.org/10.1038/sj.ijo.0802967
Shuai Z, Saremi B, Gilbert ER, Wong EA (2017) Physiological and biochemical aspects of methionine isomers and a methionine analogue in broilers. Poultry Sci 96(2):425–439. https://doi.org/10.3382/ps/pew253
Bart D, Rex GH (2001) Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr 73(6):1131S-1141S. https://doi.org/10.1556/AAlim.30.2001.2.3
Corfield AP (2015) Mucins: a biologically relevant glycan barrier in mucosal protection. Biochem Biophys Acta 1850(1):236–252. https://doi.org/10.1016/j.bbagen.2014.05.003
Young S, Mcdonald K, Newberry R, Clarke L (2021) Evaluating the role of goblet cell associated antigen passages (gaps) in the development of mucosal immune tolerance in the cftr ko intestine. Inflamm Bowel Dis 27(Supplement_1):S33-S33. https://doi.org/10.1093/ibd/izaa347.077
Knoop KA, Mcdonald KG, Mccrate S, McDole JR, Newberry RD (2015) Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol 8(1):198–210. https://doi.org/10.1038/mi.2014.58
Naegel A, Reissmann A, Wildner S, Straube S, Raithel M (2005) Evaluation of gut mucosal diamine oxidase activity (dao) in patients with food allergy and ulcerative colitis, idiopathic ulcerative colitis and Crohn’s disease. Gastroenterology 124(4):A475–A475. https://doi.org/10.1055/s-2005-921841
Zhao Y, Cao X, Fu L, Gao J (2020) N-3 pufa reduction caused by fabp2 deletion interferes with triacylglycerol metabolism and cholesterol homeostasis in fish. Appl Microbiol Biot 104(2):2149–2161. https://doi.org/10.1007/s00253-020-10366-9
Gong F, Hang YA, Huang SH, Gui CM, Chen C (2017) Effects of continuous blood purification on the inflammatory factors, endotoxin and intestinal mucosal barrier in patients with severe pancreatitis. Prog Modern Biomed 17(10):1849–1851. https://doi.org/10.13241/j.cnki.pmb.2017.10.012
Levy E, Ménard D, Delvin E, Montoudis A, Beaulieu JF, Mailhot G, Dubé N, Sinnett D, Seidman E, Bendayan M (2009) Localization, function and regulation of the two intestinal fatty acid-binding protein types. Histochem Cell Biol 132(3):351–367. https://doi.org/10.1007/s00418-009-0608-y
Al-Sadi R, Engers J, Haque M, King S, Ma TY (2021) Matrix metalloproteinase-9 (mmp-9) induced disruption of intestinal epithelial tight junction barrier is mediated by nf-κb activation. PLoS ONE 16(4):e0249544. https://doi.org/10.1371/journal.pone.0249544
Salehi P, Tafvizi F, Hesari KK (2019) Low expression of occludin in the melanoma patient. Iran J Pathol 14(4):272–278. https://doi.org/10.30699/IJP.2019.85213.1801
Muller SL, Portwich M, Schmidt A, Utepbergenov DI, Huber O, Blasig IE, Krause G (2005) The tight junction protein occludin and the adherens junction protein α-catenin share a common interaction mechanism with zo-1. J Biol Chem 280(5):3747–3756. https://doi.org/10.1074/jbc.M411365200
Fukui A, Naito Y, Handa O, Kugai M, Yoshikawa T (2012) Acetyl salicylic acid induces damage to intestinal epithelial cells by oxidation-related modifications of zo-1. Am J Physiol- Gastr L 303(8):G927–G936. https://doi.org/10.1152/ajpgi.00236.2012
Gu Z (2005) A highly specific inhibitor of matrix metalloproteinase-9 rescues laminin from proteolysis and neurons from apoptosis in transient focal cerebral ischemia. J Neurosci 25(27):6401–6408. https://doi.org/10.1523/JNEUROSCI.1563-05.2005
Poritz LS, Harris LR, Kelly AA, Koltun WA (2011) Increase in the tight junction protein claudin-1 in intestinal inflammation. Digest Dis Sci 56(10):2802–2809. https://doi.org/10.1007/s10620-011-1688-9
Hering NA, Fromm M, Schulzke J (2012) Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J Physiol 590(5):1035–1044. https://doi.org/10.1113/jphysiol.2011.224568
Acknowledgements
The authors would like to thank the co-workers of China West Normal University for their assistance in performing the experiments and analysis.
Funding
This study is supported by the program for the Fundamental Research Funds of China West Normal University (project no. 20A003), the Meritocracy Research Funds of China West Normal University (project no. 17YC349), and the Innovation Training Program for College Students (project no. S202110638058).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
All animal experiments were carried out in accordance with the Animal Welfare Committee of China West Normal University under the guidance of the Chinese government regulations.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, B., Tan, Y., Huang, H. et al. Alleviating Effect of Methionine on Intestinal Development and Intercellular Junction Induced by Nickel. Biol Trace Elem Res 200, 4007–4016 (2022). https://doi.org/10.1007/s12011-021-02992-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-02992-9