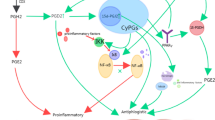
Abstract
Inflammatory bowel disease (IBD) is a chronic inflammatory condition that may emerge at a young age and often lasts for life. It often goes through phases of recurrence and remission and has a devastating effect on quality of life. The exact etiology of the disease is still unclear, but it appears that an inappropriate immune response to intestinal flora bacteria in people with a genetic predisposition may cause the disease. Managing inflammatory bowel disease is still a serious challenge. Oxidative stress and free radicals appear to be involved in the pathogenesis of this disease, and a number of studies have suggested the use of antioxidants as a therapeutic approach. The antioxidant and anti-inflammatory properties of some trace elements have led some of the research to focus on studying these trace elements in inflammatory bowel disease. Zinc and selenium are among the most important trace elements that have significant anti-inflammatory and antioxidant properties. Some studies have shown the importance of these trace elements in inflammatory bowel disease. In this review, we have attempted to provide a comprehensive overview of the findings of these studies and to gather current knowledge about the association of these trace elements with the inflammatory process and inflammatory bowel disease.

Similar content being viewed by others
Data Availability
None.
References
Molnar T, Annaházi A (2014) Pathogenesis of ulcerative colitis and Crohn’s disease: similarities, differences and a lot of things we do not know yet. J Clin Cell Immunol 5:2
Abraham C, Cho JH (2009) Inflammatory bowel disease. N Engl J Med 361:2066–2078. https://doi.org/10.1056/NEJMra0804647
Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI et al (2018) Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 390:2769–2778. https://doi.org/10.1016/s0140-6736(17)32448-0.
Naganuma M, Hosoe N, Kanai T, Ogata H (2015) Recent trends in diagnostic techniques for inflammatory bowel disease. Korean J Intern Med 30:271–278. https://doi.org/10.3904/kjim.2015.30.3.271
Moein S, Qujeq D, Vaghari Tabari M, Kashifard M, Hajian-Tilaki K (2017) Diagnostic accuracy of fecal calprotectin in assessing the severity of inflammatory bowel disease: from laboratory to clinic. Caspian J Intern Med 8:178–182. https://doi.org/10.22088/cjim.8.3.178
Johnston RD, Logan RF (2008) What is the peak age for onset of IBD? Inflamm Bowel Dis 14(Suppl 2):S4–S5. https://doi.org/10.1002/ibd.20545
Devlen J, Beusterien K, Yen L, Ahmed A, Cheifetz AS, Moss AC (2014) The burden of inflammatory bowel disease: a patient-reported qualitative analysis and development of a conceptual model. Inflamm Bowel Dis 20:545–552. https://doi.org/10.1097/01.mib.0000440983.86659.81
Piechota-Polanczyk A, Fichna J (2014) Review article: the role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch Pharmacol 387:605–620. https://doi.org/10.1007/s00210-014-0985-1
Vaghari-Tabari M, Moein S, Qujeq D, Kashifard M, Hajian-Tilaki K (2018) Positive correlation of fecal calprotectin with serum antioxidant enzymes in patients with inflammatory bowel disease: accidental numerical correlation or a new finding? Am J Med Sci 355:449–455. https://doi.org/10.1016/j.amjms.2017.12.009
Vaghari Tabari M, Moein S, Qujeq D, Kashifard M, Shokri Shirvani J, Hajian Tilaki K et al (2017) Evaluation of the potential antioxidant role of high-density lipoprotein-cholesterol (HDL-C) in patients with ulcerative colitis. Ann Colorectal Res 5:e13699. https://doi.org/10.5812/acr.13699
Moura FA, de Andrade KQ, Dos Santos JCF, Araujo ORP, Goulart MOF (2015) Antioxidant therapy for treatment of inflammatory bowel disease: does it work? Redox Biol 6:617–639. https://doi.org/10.1016/j.redox.2015.10.006
Choi R, Sun J, Yoo H, Kim S, Cho YY, Kim HJ, Kim S, Chung J, Oh SY, Lee SY (2016) A prospective study of serum trace elements in healthy Korean pregnant women. Nutrients 8. https://doi.org/10.3390/nu8110749
Bhattacharya PT, Misra SR, Hussain M (2016) Nutritional aspects of essential trace elements in oral health and disease: an extensive review. Scientifica (Cairo) 2016:5464373. https://doi.org/10.1155/2016/5464373
Goldhaber SB (2003) Trace element risk assessment: essentiality vs. toxicity. Regul Toxicol Pharmacol 38:232–242
Sapkota M, Knoell DL (2018) Essential role of zinc and zinc transporters in myeloid cell function and host defense against infection. J Immunol Res 2018:4315140. https://doi.org/10.1155/2018/4315140
Escobedo Monge MF, Barrado E, Alonso Vicente C, Redondo Del Rio MP, Marugan de Miguelsanz JM (2019) Zinc nutritional status in patients with cystic fibrosis. Nutrients 11. https://doi.org/10.3390/nu11010150
Evans GW (1986) Zinc and its deficiency diseases. Clin Physiol Biochem 4:94–98
Gammoh NZ, Rink L (2017) Zinc in infection and inflammation. Nutrients 9. https://doi.org/10.3390/nu9060624
Gilca-Blanariu GE, Diaconescu S, Ciocoiu M, Stefanescu G (2018) New insights into the role of trace elements in IBD. Biomed Res Int 2018:1813047. https://doi.org/10.1155/2018/1813047
Stoffaneller R, Morse NL (2015) A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients. 7:1494–1537. https://doi.org/10.3390/nu7031494
Avery JC, Hoffmann PR (2018) Selenium, selenoproteins, and immunity. Nutrients 10. https://doi.org/10.3390/nu10091203
Bera S, De Rosa V, Rachidi W, Diamond AM (2013) Does a role for selenium in DNA damage repair explain apparent controversies in its use in chemoprevention? Mutagenesis. 28:127–134. https://doi.org/10.1093/mutage/ges064
Zeng H (2009) Selenium as an essential micronutrient: roles in cell cycle and apoptosis. Molecules. 14:1263–1278. https://doi.org/10.3390/molecules14031263
Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E (2006) Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr 84:762–773. https://doi.org/10.1093/ajcn/84.4.762
Jaworska K, Gupta S, Durda K, Muszynska M, Sukiennicki G, Jaworowska E et al (2013) A low selenium level is associated with lung and laryngeal cancers. PLoS One 8:e59051. https://doi.org/10.1371/journal.pone.0059051
Pizzulli A, Ranjbar A (2000) Selenium deficiency and hypothyroidism: a new etiology in the differential diagnosis of hypothyroidism in children. Biol Trace Elem Res 77:199–208. https://doi.org/10.1385/bter:77:3:199
Fritz H, Kennedy D, Fergusson D, Fernandes R, Cooley K, Seely A et al (2011) Selenium and lung cancer: a systematic review and meta analysis. PLoS One 6:e26259. https://doi.org/10.1371/journal.pone.0026259
Vinceti M, Filippini T, Del Giovane C, Dennert G, Zwahlen M, Brinkman M et al (2018) Selenium for preventing cancer. Cochrane Database Syst Rev 1:CD005195. https://doi.org/10.1002/14651858.CD005195.pub4.
Castro Aguilar-Tablada T, Navarro-Alarcon M, Quesada Granados J, Samaniego Sanchez C, Rufian-Henares JA, Nogueras-Lopez F (2016) Ulcerative colitis and Crohn's disease are associated with decreased serum selenium concentrations and increased cardiovascular risk. Nutrients 8. https://doi.org/10.3390/nu8120780
Moein S, Vaghari-Tabari M, Qujeq D, Majidinia M, Nabavi SM, Yousefi B (2019) MiRNAs and inflammatory bowel disease: an interesting new story. J Cell Physiol 234:3277–3293. https://doi.org/10.1002/jcp.27173
Hendrickson BA, Gokhale R, Cho JH (2002) Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev 15:79–94. https://doi.org/10.1128/cmr.15.1.79-94.2002
Tulewicz-Marti E, Moniuszko A, Rydzewska G (2017) Management of anemia in inflammatory bowel disease: a challenge in everyday clinical practice. Prz Gastroenterol 12:239–243. https://doi.org/10.5114/pg.2017.72096
Manganiotis AN, Banner MP, Malkowicz SB (2001) Urologic complications of Crohn's disease. Surg Clin North Am 81:197–215, x. https://doi.org/10.1016/s0039-6109(05)70281-4
Bopanna S, Ananthakrishnan AN, Kedia S, Yajnik V, Ahuja V (2017) Risk of colorectal cancer in Asian patients with ulcerative colitis: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2:269–276. https://doi.org/10.1016/s2468-1253(17)30004-3
Ng WK, Wong SH, Ng SC (2016) Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res 14:111–119. https://doi.org/10.5217/ir.2016.14.2.111
Kaser A, Zeissig S, Blumberg RS (2010) Inflammatory bowel disease. Annu Rev Immunol 28:573–621. https://doi.org/10.1146/annurev-immunol-030409-101225
Guan G, Lan S (2018) Implications of antioxidant systems in inflammatory bowel disease. Biomed Res Int 2018:1290179. https://doi.org/10.1155/2018/1290179
Tambuwala MM (2016) Natural nuclear factor kappa beta inhibitors: safe therapeutic options for inflammatory bowel disease. Inflamm Bowel Dis 22:719–723. https://doi.org/10.1097/mib.0000000000000655
Rosenfeld G, Bressler B (2012) The truth about cigarette smoking and the risk of inflammatory bowel disease. Am J Gastroenterol 107:1407–1408. https://doi.org/10.1038/ajg.2012.190.
Ananthakrishnan AN, Khalili H, Song M, Higuchi LM, Richter JM, Chan AT (2015) Zinc intake and risk of Crohn's disease and ulcerative colitis: a prospective cohort study. Int J Epidemiol 44:1995–2005. https://doi.org/10.1093/ije/dyv301
Ak T, Gülçin I (2008) Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 174:27–37. https://doi.org/10.1016/j.cbi.2008.05.003
Gülçin I (2009) Antioxidant activity of L-adrenaline: a structure-activity insight. Chem Biol Interact 179:71–80. https://doi.org/10.1016/j.cbi.2008.09.023
Gülçin İ (2012) Antioxidant activity of food constituents: an overview. Arch Toxicol 86:345–391. https://doi.org/10.1007/s00204-011-0774-2
Gülçin İ (2010) Antioxidant properties of resveratrol: a structure–activity insight. Innovative Food Sci Emerg Technol 11:210–218. https://doi.org/10.1016/j.ifset.2009.07.002
Gülçin İ (2011) Antioxidant activity of eugenol: a structure-activity relationship study. J Med Food 14:975–985. https://doi.org/10.1089/jmf.2010.0197
Taslimi P, Gulçin İ (2018) Antioxidant and anticholinergic properties of olivetol. J Food Biochem 42:e12516. https://doi.org/10.1111/jfbc.12516
Gulcin İ (2020) Antioxidants and antioxidant methods: an updated overview. Arch Toxicol 94:651–715. https://doi.org/10.1007/s00204-020-02689-3
Balmus IM, Ciobica A, Trifan A, Stanciu C (2016) The implications of oxidative stress and antioxidant therapies in inflammatory bowel disease: clinical aspects and animal models. Saudi J Gastroenterol 22:3–17. https://doi.org/10.4103/1319-3767.173753
Phaniendra A, Jestadi DB, Periyasamy L (2015) Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem 30:11–26. https://doi.org/10.1007/s12291-014-0446-0
Wong-Ekkabut J, Xu Z, Triampo W, Tang IM, Tieleman DP, Monticelli L (2007) Effect of lipid peroxidation on the properties of lipid bilayers: a molecular dynamics study. Biophys J 93:4225–4236. https://doi.org/10.1529/biophysj.107.112565
Achitei D, Ciobica A, Balan G, Gologan E, Stanciu C, Stefanescu G (2013) Different profile of peripheral antioxidant enzymes and lipid peroxidation in active and non-active inflammatory bowel disease patients. Dig Dis Sci 58:1244–1249. https://doi.org/10.1007/s10620-012-2510-z
Yasukawa K, Tokuda H, Tun X, Utsumi H, Yamada K (2012) The detrimental effect of nitric oxide on tissue is associated with inflammatory events in the vascular endothelium and neutrophils in mice with dextran sodium sulfate-induced colitis. Free Radic Res 46:1427–1436. https://doi.org/10.3109/10715762.2012.732698
Al-Sadi R, Guo S, Ye D, Ma TY (2013) TNF-alpha modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am J Pathol 183:1871–1884. https://doi.org/10.1016/j.ajpath.2013.09.001
Kuwano Y, Tominaga K, Kawahara T, Sasaki H, Takeo K, Nishida K et al (2008) Tumor necrosis factor alpha activates transcription of the NADPH oxidase organizer 1 (NOXO1) gene and upregulates superoxide production in colon epithelial cells. Free Radic Biol Med 45:1642–1652. https://doi.org/10.1016/j.freeradbiomed.2008.08.033
Rao RK, Basuroy S, Rao VU, Karnaky KJ Jr, Gupta A (2002) Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J 368:471–481. https://doi.org/10.1042/bj20011804
Baranipour S, Amini Kadijani A, Qujeq D, Shahrokh S, Haghazali M, Mirzaei A et al (2018) Inducible nitric oxide synthase as a potential blood-based biomarker in inflammatory bowel diseases. Gastroenterol Hepatol Bed Bench 11:S124–S1S8
Reimund JM, Allison AC, Muller CD, Dumont S, Kenney JS, Baumann R et al (1998) Antioxidants inhibit the in vitro production of inflammatory cytokines in Crohn's disease and ulcerative colitis. Eur J Clin Investig 28:145–150. https://doi.org/10.1046/j.1365-2362.1998.00257.x
Sutti S, Jindal A, Locatelli I, Vacchiano M, Gigliotti L, Bozzola C et al (2014) Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology. 59:886–897. https://doi.org/10.1002/hep.26749
Schreck R, Rieber P, Baeuerle PA (1991) Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J 10:2247–2258
Pan H, Wang H, Wang X, Zhu L, Mao L (2012) The absence of Nrf2 enhances NF-kappaB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediat Inflamm 2012:217580. https://doi.org/10.1155/2012/217580
Anrather J, Racchumi G, Iadecola C (2006) NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem 281:5657–5667. https://doi.org/10.1074/jbc.M506172200
Morgan MJ, Liu ZG (2011) Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res 21:103–115. https://doi.org/10.1038/cr.2010.178
Tuzun A, Erdil A, Inal V, Aydin A, Bagci S, Yesilova Z et al (2002) Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin Biochem 35:569–572
Sido B, Hack V, Hochlehnert A, Lipps H, Herfarth C, Droge W (1998) Impairment of intestinal glutathione synthesis in patients with inflammatory bowel disease. Gut. 42:485–492. https://doi.org/10.1136/gut.42.4.485
Kuroki F, Iida M, Tominaga M, Matsumoto T, Hirakawa K, Sugiyama S et al (1993) Multiple vitamin status in Crohn's disease. Correlation with disease activity. Dig Dis Sci 38:1614–1618. https://doi.org/10.1007/bf01303168
Isik B, Ceylan A, Isik R (2007) Oxidative stress in smokers and non-smokers. Inhal Toxicol 19:767–769. https://doi.org/10.1080/08958370701401418
Mangiapane E, Pessione A, Pessione E (2014) Selenium and selenoproteins: an overview on different biological systems. Curr Protein Pept Sci 15:598–607. https://doi.org/10.2174/1389203715666140608151134
Tinggi U (2008) Selenium: its role as antioxidant in human health. Environ Health Prev Med 13:102–108. https://doi.org/10.1007/s12199-007-0019-4
Stadtman TC (2005) Selenoproteins--tracing the role of a trace element in protein function. PLoS Biol 3:e421. https://doi.org/10.1371/journal.pbio.0030421
Han YM, Yoon H, Lim S, Sung MK, Shin CM, Park YS et al (2017) Risk factors for vitamin D, zinc, and selenium deficiencies in Korean patients with inflammatory bowel disease. Gut Liver 11:363–369. https://doi.org/10.5009/gnl16333
Labunskyy VM, Hatfield DL, Gladyshev VN (2014) Selenoproteins: molecular pathways and physiological roles. Physiol Rev 94:739–777. https://doi.org/10.1152/physrev.00039.2013
Kudva AK, Shay AE, Prabhu KS (2015) Selenium and inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol 309:G71–G77. https://doi.org/10.1152/ajpgi.00379.2014
Tian T, Wang Z, Zhang J (2017) Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxidative Med Cell Longev 2017:4535194. https://doi.org/10.1155/2017/4535194
Combs GF Jr, Watts JC, Jackson MI, Johnson LK, Zeng H, Scheett AJ et al (2011) Determinants of selenium status in healthy adults. Nutr J 10:75. https://doi.org/10.1186/1475-2891-10-75
Esworthy RS, Aranda R, Martin MG, Doroshow JH, Binder SW, Chu FF (2001) Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am J Physiol Gastrointest Liver Physiol 281:G848–G855. https://doi.org/10.1152/ajpgi.2001.281.3.G848
Krehl S, Loewinger M, Florian S, Kipp AP, Banning A, Wessjohann LA et al (2012) Glutathione peroxidase-2 and selenium decreased inflammation and tumors in a mouse model of inflammation-associated carcinogenesis whereas sulforaphane effects differed with selenium supply. Carcinogenesis. 33:620–628. https://doi.org/10.1093/carcin/bgr288
Barrett CW, Ning W, Chen X, Smith JJ, Washington MK, Hill KE et al (2013) Tumor suppressor function of the plasma glutathione peroxidase gpx3 in colitis-associated carcinoma. Cancer Res 73:1245–1255. https://doi.org/10.1158/0008-5472.can-12-3150
Nunes C, Teixeira N, Serra D, Freitas V, Almeida L, Laranjinha J (2016) Red wine polyphenol extract efficiently protects intestinal epithelial cells from inflammation via opposite modulation of JAK/STAT and Nrf2 pathways. Toxicol Res (Camb) 5:53–65. https://doi.org/10.1039/c5tx00214a
Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN (2006) Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res 66:11580–11584. https://doi.org/10.1158/0008-5472.can-06-3562
Cebula M, Schmidt EE, Arner ES (2015) TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxid Redox Signal 23:823–853. https://doi.org/10.1089/ars.2015.6378
Brigelius-Flohe R, Muller M, Lippmann D, Kipp AP (2012) The yin and yang of nrf2-regulated selenoproteins in carcinogenesis. Int J Cell Biol 2012:486147. https://doi.org/10.1155/2012/486147
Reszka E, Wieczorek E, Jablonska E, Janasik B, Fendler W, Wasowicz W (2015) Association between plasma selenium level and NRF2 target genes expression in humans. J Trace Elem Med Biol 30:102–106. https://doi.org/10.1016/j.jtemb.2014.11.008
Prabhu KS, Zamamiri-Davis F, Stewart JB, Thompson JT, Sordillo LM, Reddy CC (2002) Selenium deficiency increases the expression of inducible nitric oxide synthase in RAW 264.7 macrophages: role of nuclear factor-kappaB in up-regulation. Biochem J 366:203–209. https://doi.org/10.1042/bj20020256
Kim SH, Johnson VJ, Shin TY, Sharma RP (2004) Selenium attenuates lipopolysaccharide-induced oxidative stress responses through modulation of p38 MAPK and NF-kappaB signaling pathways. Exp Biol Med (Maywood) 229:203–213. https://doi.org/10.1177/153537020422900209
Zhu C, Zhang S, Song C, Zhang Y, Ling Q, Hoffmann PR et al (2017) Selenium nanoparticles decorated with Ulva lactuca polysaccharide potentially attenuate colitis by inhibiting NF-kappaB mediated hyper inflammation. J Nanobiotechnology 15:20. https://doi.org/10.1186/s12951-017-0252-y
Nelson SM, Lei X, Prabhu KS (2011) Selenium levels affect the IL-4-induced expression of alternative activation markers in murine macrophages. J Nutr 141:1754–1761. https://doi.org/10.3945/jn.111.141176
Abron JD, Singh NP, Price RL, Nagarkatti M, Nagarkatti PS, Singh UP (2018) Genistein induces macrophage polarization and systemic cytokine to ameliorate experimental colitis. PLoS One 13:e0199631. https://doi.org/10.1371/journal.pone.0199631
Vunta H, Belda BJ, Arner RJ, Channa Reddy C, Vanden Heuvel JP, Sandeep Prabhu K (2008) Selenium attenuates pro-inflammatory gene expression in macrophages. Mol Nutr Food Res 52:1316–1323. https://doi.org/10.1002/mnfr.200700346
Kaushal N, Kudva AK, Patterson AD, Chiaro C, Kennett MJ, Desai D et al (2014) Crucial role of macrophage selenoproteins in experimental colitis. J Immunol 193:3683–3692. https://doi.org/10.4049/jimmunol.1400347
Gandhi UH, Kaushal N, Ravindra KC, Hegde S, Nelson SM, Narayan V et al (2011) Selenoprotein-dependent up-regulation of hematopoietic prostaglandin D2 synthase in macrophages is mediated through the activation of peroxisome proliferator-activated receptor (PPAR) gamma. J Biol Chem 286:27471–27482. https://doi.org/10.1074/jbc.M111.260547
Vong L, Ferraz JG, Panaccione R, Beck PL, Wallace JL (2010) A pro-resolution mediator, prostaglandin D(2), is specifically up-regulated in individuals in long-term remission from ulcerative colitis. Proc Natl Acad Sci U S A 107:12023–12027. https://doi.org/10.1073/pnas.1004982107
Choi JM, Bothwell AL (2012) The nuclear receptor PPARs as important regulators of T-cell functions and autoimmune diseases. Mol Cell 33:217–222. https://doi.org/10.1007/s10059-012-2297-y
Le Loupp AG, Bach-Ngohou K, Bourreille A, Boudin H, Rolli-Derkinderen M, Denis MG et al (2015) Activation of the prostaglandin D2 metabolic pathway in Crohn's disease: involvement of the enteric nervous system. BMC Gastroenterol 15:112. https://doi.org/10.1186/s12876-015-0338-7
Choo J, Lee Y, Yan XJ, Noh TH, Kim SJ, Son S et al (2015) A novel peroxisome proliferator-activated receptor (PPAR)gamma agonist 2-hydroxyethyl 5-chloro-4,5-didehydrojasmonate exerts anti-inflammatory effects in colitis. J Biol Chem 290:25609–25619. https://doi.org/10.1074/jbc.M115.673046
Sheibanie AF, Yen JH, Khayrullina T, Emig F, Zhang M, Tuma R et al (2007) The proinflammatory effect of prostaglandin E2 in experimental inflammatory bowel disease is mediated through the IL-23-- > IL-17 axis. J Immunol 178:8138–8147. https://doi.org/10.4049/jimmunol.178.12.8138
Kaur R, Thakur S, Rastogi P, Kaushal N (2018) Resolution of Cox mediated inflammation by Se supplementation in mouse experimental model of colitis. PLoS One 13:e0201356. https://doi.org/10.1371/journal.pone.0201356
Dhanjal NI, Sharma S, Skalny AV, Skalnaya MG, Ajsuvakova OP, Tinkov AA et al (2019) Selenium-rich maize modulates the expression of prostaglandin genes in lipopolysaccharide-stimulated RAW264.7 macrophages. Food Funct 10:2839–2846. https://doi.org/10.1039/c9fo00186g
Kim W, Lee Y, Jeong S, Nam J, Lee S, Jung Y (2015) Colonic delivery of celecoxib is a potential pharmaceutical strategy for repositioning the selective COX-2 inhibitor as an anti-colitic agent. Arch Pharm Res 38:1830–1838. https://doi.org/10.1007/s12272-015-0602-y
Sang L, Chang B, Zhu J, Yang F, Li Y, Jiang X et al (2016) Dextran sulfate sodium-induced acute experimental colitis in C57BL/6 mice is mitigated by selenium. Int Immunopharmacol 39:359–368. https://doi.org/10.1016/j.intimp.2016.07.034
Sang LX, Chang B, Zhu JF, Yang FL, Li Y, Jiang XF et al (2017) Sodium selenite ameliorates dextran sulfate sodium-induced chronic colitis in mice by decreasing Th1, Th17, and gammadeltaT and increasing CD4(+)CD25(+) regulatory T-cell responses. World J Gastroenterol 23:3850–3863. https://doi.org/10.3748/wjg.v23.i21.3850
Thorsteinsdottir S, Gudjonsson T, Nielsen OH, Vainer B, Seidelin JB (2011) Pathogenesis and biomarkers of carcinogenesis in ulcerative colitis. Nat Rev Gastroenterol Hepatol 8:395–404. https://doi.org/10.1038/nrgastro.2011.96
El-Demerdash FM, Nasr HM (2014) Antioxidant effect of selenium on lipid peroxidation, hyperlipidemia and biochemical parameters in rats exposed to diazinon. J Trace Elem Med Biol 28:89–93. https://doi.org/10.1016/j.jtemb.2013.10.001
Akil M, Bicer M, Menevse E, Baltaci AK, Mogulkoc R (2011) Selenium supplementation prevents lipid peroxidation caused by arduous exercise in rat brain tissue. Bratisl Lek Listy 112:314–317
Short SP, Pilat JM, Williams CS (2018) Roles for selenium and selenoprotein P in the development, progression, and prevention of intestinal disease. Free Radic Biol Med 127:26–35. https://doi.org/10.1016/j.freeradbiomed.2018.05.066
Bjornstedt M, Hamberg M, Kumar S, Xue J, Holmgren A (1995) Human thioredoxin reductase directly reduces lipid hydroperoxides by NADPH and selenocystine strongly stimulates the reaction via catalytically generated selenols. J Biol Chem 270:11761–11764. https://doi.org/10.1074/jbc.270.20.11761
Rock C, Moos PJ (2010) Selenoprotein P protects cells from lipid hydroperoxides generated by 15-LOX-1. Prostaglandins Leukot Essent Fat Acids 83:203–210. https://doi.org/10.1016/j.plefa.2010.08.006
Lee HH, Prasad AS, Brewer GJ, Owyang C (1989) Zinc absorption in human small intestine. Am J Physiol 256:G87–G91
Prasad AS (2014) Zinc is an antioxidant and anti-inflammatory agent: its role in human health. Front Nutr 1:14
Siva S, Rubin DT, Gulotta G, Wroblewski K, Pekow J (2016) Zinc deficiency is associated with poor clinical outcomes in patients with inflammatory bowel disease. Inflamm Bowel Dis 23:152–157
Vagianos K, Bector S, McConnell J, Bernstein CN (2007) Nutrition assessment of patients with inflammatory bowel disease. J Parenter Enter Nutr 31:311–319
Alkhouri RH, Hashmi H, Baker RD, Gelfond D, Baker SS (2013) Vitamin and mineral status in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 56:89–92
Skrovanek S, DiGuilio K, Bailey R, Huntington W, Urbas R, Mayilvaganan B et al (2014) Zinc and gastrointestinal disease. World J Gastrointest Pathophysiol 5:496
Ranaldi G, Ferruzza S, Canali R, Leoni G, Zalewski PD, Sambuy Y et al (2013) Intracellular zinc is required for intestinal cell survival signals triggered by the inflammatory cytokine TNFα. J Nutr Biochem 24:967–976
Mayer LS, Uciechowski P, Meyer S, Schwerdtle T, Rink L, Haase H (2014) Differential impact of zinc deficiency on phagocytosis, oxidative burst, and production of pro-inflammatory cytokines by human monocytes. Metallomics. 6:1288–1295
Kitabayashi C, Fukada T, Kanamoto M, Ohashi W, Hojyo S, Atsumi T et al (2010) Zinc suppresses Th17 development via inhibition of STAT3 activation. Int Immunol 22:375–386. https://doi.org/10.1093/intimm/dxq017
Mohammadi E, Qujeq D, Taheri H, Hajian-Tilaki K (2017) Evaluation of serum trace element levels and superoxide dismutase activity in patients with inflammatory bowel disease: translating basic research into clinical application. Biol Trace Elem Res 177:235–240
Boztaş M, Çetinkaya Y, Topal M (2015) Gülçin İ, Menzek A, Şahin E, et al. Synthesis and carbonic anhydrase isoenzymes I, II, IX, and XII inhibitory effects of dimethoxybromophenol derivatives incorporating cyclopropane moieties. J Med Chem 58:640–650. https://doi.org/10.1021/jm501573b.
Arabaci B, Gulcin I, Alwasel S (2014) Capsaicin: a potent inhibitor of carbonic anhydrase isoenzymes. Molecules. 19:10103–10114. https://doi.org/10.3390/molecules190710103
Topal F, Gulcin I, Dastan A, Guney M (2017) Novel eugenol derivatives: potent acetylcholinesterase and carbonic anhydrase inhibitors. Int J Biol Macromol 94:845–851. https://doi.org/10.1016/j.ijbiomac.2016.10.096
Gülçin İ, Trofimov B, Kaya R, Taslimi P, Sobenina L, Schmidt E et al (2020) Synthesis of nitrogen, phosphorus, selenium and sulfur-containing heterocyclic compounds - determination of their carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase and α-glycosidase inhibition properties. Bioorg Chem 103:104171. https://doi.org/10.1016/j.bioorg.2020.104171
Oteiza PI (2012) Zinc and the modulation of redox homeostasis. Free Radic Biol Med 53:1748–1759
Ruz M, Carrasco F, Rojas P, Codoceo J, Inostroza J, Basfi-Fer K et al (2013) Zinc as a potential coadjuvant in therapy for type 2 diabetes. Food Nutr Bull 34:215–221
Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME (2012) Zinc and human health: an update. Arch Toxicol 86:521–534
Sato M, Bremner I (1993) Oxygen free radicals and metallothionein. Free Radic Biol Med 14:325–337
Bogani D, Morgan MA, Nelson AC, Costello I, McGouran JF, Kessler BM et al (2013) The PR/SET domain zinc finger protein Prdm4 regulates gene expression in embryonic stem cells but plays a nonessential role in the developing mouse embryo. Mol Cell Biol 33:3936–3950
Alam S, Kelleher SL (2012) Cellular mechanisms of zinc dysregulation: a perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients. 4:875–903
Choi S, Liu X, Pan Z (2018) Zinc deficiency and cellular oxidative stress: prognostic implications in cardiovascular diseases. Acta Pharmacol Sin 39:1120
Tsuji T, Naito Y, Takagi T, Kugai M, Yoriki H, Horie R et al (2013) Role of metallothionein in murine experimental colitis. Int J Mol Med 31:1037–1046
Westin G, Schaffner W (1988) A zinc-responsive factor interacts with a metal-regulated enhancer element (MRE) of the mouse metallothionein-I gene. EMBO J 7:3763–3770
Itoh N, Shibayama H, Kanekiyo M, Namphung D, Nakanishi T, Matsuyama A et al (2005) Reduced bactericidal activity and nitric oxide production in metallothionein-deficient macrophages in response to lipopolysaccharide stimulation. Toxicology. 216:188–196
Mulder T, Veer AS, Verspaget H, Griffioen G, Peña A, Janssens A et al (1994) Effect of oral zinc supplementation on metallothionein and superoxide dismutase concentrations in patients with inflammatory bowel disease. J Gastroenterol Hepatol 9:472–477
Di Leo V, D'Incà R, Barollo M, Tropea A, Fries W, Mazzon E et al (2001) Effect of zinc supplementation on trace elements and intestinal metallothionein concentrations in experimental colitis in the rat. Dig Liver Dis 33:135–139
Tran C, Ball J, Sundar S, Coyle P, Howarth G (2007) The role of zinc and metallothionein in the dextran sulfate sodium-induced colitis mouse model. Dig Dis Sci 52:2113–2121
Tran C, Butler R, Philcox J, Rofe A, Howarth G, Coyle P (1998) Regional distribution of metallothionein and zinc in the mouse gut. Biol Trace Elem Res 63:239–251
Kruidenier L, Kuiper I, Lamers CB, Verspaget HW (2003) Intestinal oxidative damage in inflammatory bowel disease: semi-quantification, localization, and association with mucosal antioxidants. J Pathol 201:28–36
Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E et al (1996) Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci 41:2078–2086
Kruidenier L, Kuiper I, van Duijn W, Mieremet-Ooms MA, van Hogezand RA, Lamers CB et al (2003) Imbalanced secondary mucosal antioxidant response in inflammatory bowel disease. J Pathol 201:17–27
Seguí J, Gironella M, Sans M, Granell S, Gil F, Gimeno M et al (2004) Superoxide dismutase ameliorates TNBS-induced colitis by reducing oxidative stress, adhesion molecule expression, and leukocyte recruitment into the inflamed intestine. J Leukoc Biol 76:537–544
Matias JP, Costa DM, Cruz KJC, Silva KG, Feitosa MM, Medeiros LGO et al (2015) Effect of zinc supplementation on superoxide dismutase activity in patients with ulcerative rectocolitis. Nutr Hosp 31:1434–1437
Bedard K, Krause K-H (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313
Szanto I, Rubbia-Brandt L, Kiss P, Steger K, Banfi B, Kovari E et al (2005) Expression of NOX1, a superoxide-generating NADPH oxidase, in colon cancer and inflammatory bowel disease. J Pathol 207:164–176
Ramonaite R, Skieceviciene J, Kiudelis G, Jonaitis L, Tamelis A, Cizas P et al (2013) Influence of NADPH oxidase on inflammatory response in primary intestinal epithelial cells in patients with ulcerative colitis. BMC Gastroenterol 13:159
Kim J, Cha Y-N, Surh Y-J (2010) A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res 690:12–23
Khor TO, Huang M-T, Prawan A, Liu Y, Hao X, Yu S et al (2008) Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prev Res 1:187–191
Lee K-M, Kang K, Lee SB, Nho CW (2013) Nuclear factor-E2 (Nrf2) is regulated through the differential activation of ERK1/2 and PKC α/βII by Gymnasterkoreayne B. Cancer Lett 330:225–232
Li J, Chen H, Wang B, Cai C, Yang X, Chai Z et al (2017) ZnO nanoparticles act as supportive therapy in DSS-induced ulcerative colitis in mice by maintaining gut homeostasis and activating Nrf2 signaling. Sci Rep 7:43126
Gîlcă-Blanariu G-E, Diaconescu S, Ciocoiu M, Ștefănescu G (2018) New insights into the role of trace elements in IBD. Biomed Res Int 2018:1813047.
Lee H, Kim B, Choi YH, Hwang Y, Kim DH, Cho S et al (2015) Inhibition of interleukin-1beta-mediated interleukin-1 receptor-associated kinase 4 phosphorylation by zinc leads to repression of memory T helper type 17 response in humans. Immunology. 146:645–656. https://doi.org/10.1111/imm.12536
Chiba H, Kojima T, Osanai M, Sawada N (2006) The significance of interferon-gamma-triggered internalization of tight-junction proteins in inflammatory bowel disease. Sci STKE 2006:pe1. https://doi.org/10.1126/stke.3162006pe1
Hayashi K, Ishizuka S, Yokoyama C, Hatae T (2008) Attenuation of interferon-gamma mRNA expression in activated Jurkat T cells by exogenous zinc via down-regulation of the calcium-independent PKC-AP-1 signaling pathway. Life Sci 83:6–11. https://doi.org/10.1016/j.lfs.2008.04.022
Wong CP, Rinaldi NA, Ho E (2015) Zinc deficiency enhanced inflammatory response by increasing immune cell activation and inducing IL6 promoter demethylation. Mol Nutr Food Res 59:991–999. https://doi.org/10.1002/mnfr.201400761
Rosenkranz E, Maywald M, Hilgers RD, Brieger A, Clarner T, Kipp M et al (2016) Induction of regulatory T cells in Th1-/Th17-driven experimental autoimmune encephalomyelitis by zinc administration. J Nutr Biochem 29:116–123. https://doi.org/10.1016/j.jnutbio.2015.11.010
Ueno A, Ghosh A, Hung D, Li J, Jijon H (2015) Th17 plasticity and its changes associated with inflammatory bowel disease. World J Gastroenterol 21:12283–12295. https://doi.org/10.3748/wjg.v21.i43.12283
Kido T, Ishiwata K, Suka M, Yanagisawa H (2019) Inflammatory response under zinc deficiency is exacerbated by dysfunction of the T helper type 2 lymphocyte-M2 macrophage pathway. Immunology. 156:356–372. https://doi.org/10.1111/imm.13033
Kilby K, Mathias H, Boisvenue L, Heisler C, Jones JL (2019) Micronutrient absorption and related outcomes in people with inflammatory bowel disease: a review. Nutrients. 11:1388
Ma A, Malynn BA (2012) A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol 12:774
Morgan CI, Ledford JR, Zhou P, Page K (2011) Zinc supplementation alters airway inflammation and airway hyperresponsiveness to a common allergen. J Inflamm 8:36
Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T (2017) Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 25:11–24
Liu M-J, Bao S, Gálvez-Peralta M, Pyle CJ, Rudawsky AC, Pavlovicz RE et al (2013) The zinc transporter SLC39A8 is a negative feedback regulator of NF-κB through zinc-mediated inhibition of IKK. Cell Rep 3:386
Miyoshi Y, Tanabe S, Suzuki T (2016) Cellular zinc is required for intestinal epithelial barrier maintenance via the regulation of claudin-3 and occludin expression. Am J Physiol Gastrointest Liver Physiol 311:G105–G116. https://doi.org/10.1152/ajpgi.00405.2015
Younus MM, Taher MA, Askar BA, Kurmanji JM (2015) Serum selenium correlations with C-reactive protein, serum calprotectin and disease activity in IBD patients treated with infliximab. World J Pharm Pharm Sci 4(9):165–179
Gentschew L, Bishop KS, Han DY, Morgan AR, Fraser AG, Lam WJ et al (2012) Selenium, selenoprotein genes and Crohn's disease in a case-control population from Auckland, New Zealand. Nutrients. 4:1247–1259. https://doi.org/10.3390/nu4091247
Poursadegh F, Ahadi M, Vosoughinia H, Salehi M, Beheshti Namdar A, Farzanehfar MR et al (2018) A STROBE compliant observational study on trace elements in patients with ulcerative colitis and their relationship with disease activity. Medicine (Baltimore) 97:e13523. https://doi.org/10.1097/md.0000000000013523
Stedman JD, Spyrou NM, Millar AD, Altaf WJ, Akanle OA, Rampton DS (1997) Selenium supplementation in the diets of patients suffering from ulcerative colitis. J Radioanal Nucl Chem 217:189–191. https://doi.org/10.1007/BF02034441
Younus MM, Taher MA, ALMaliki JM, AlKhalidi NM (2015) Selenium supplementation may decrease the rate of infliximab ADRS in IBD patients. World J Pharm Pharm Sci 4(9):193–203
Stochel-Gaudyn A, Fyderek K, Koscielniak P (2019) Serum trace elements profile in the pediatric inflammatory bowel disease progress evaluation. J Trace Elem Med Biol 55:121–126. https://doi.org/10.1016/j.jtemb.2019.06.016
Kobayashi Y, Ohfuji S, Kondo K, Fukushima W, Sasaki S, Kamata N et al (2019) Association between dietary iron and zinc intake and development of ulcerative colitis: a case-control study in Japan. J Gastroenterol Hepatol. https://doi.org/10.1111/jgh.14642
Mechie NC, Mavropoulou E, Ellenrieder V, Petzold G, Kunsch S, Neesse A et al (2019) Serum vitamin D but not zinc levels are associated with different disease activity status in patients with inflammatory bowel disease. Medicine (Baltimore) 98:e15172. https://doi.org/10.1097/md.0000000000015172
Acknowledgments
We are very grateful to Prof. Gordon Ferns for his helpful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vaghari-Tabari, M., Jafari-Gharabaghlou, D., Sadeghsoltani, F. et al. Zinc and Selenium in Inflammatory Bowel Disease: Trace Elements with Key Roles?. Biol Trace Elem Res 199, 3190–3204 (2021). https://doi.org/10.1007/s12011-020-02444-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02444-w