Abstract
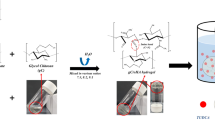
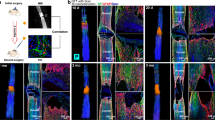
The purpose of this study was to evaluate the neuroprotective effect of local implantation of a controlled delivery system of chitosan hydrogel loaded with selenium nanoparticles in rats with spinal cord injury (SCI). For this purpose, 60 adult female rats were randomly divided into three equal groups. In all three groups, SCI was induced by aneurysm clamping at the level of thoracic vertebrae under inhaled anesthesia with isoflurane. In one group after spinal cord injury, chitosan hydrogels loaded with selenium nanoparticles (treatment group), and in the other group, only chitosan hydrogels (positive control group) were placed at the site of injury. In the last group (negative control), no material was placed in the injury site. Hematoxylin-eosin and glial fibrillary acidic protein (GFAP) staining evaluated histological changes at the site of injury on days 3, 7, 21, and 28 after surgery. Evaluations show that hemorrhage and inflammation also have a marked decrease in inflammatory cells at different times in the treatment group. This decrease was also seen in the chitosan group but was less severe than in the treatment group. The formation of nerve fibers was also observed in the treatment group over time of injury. Immunohistochemical studies of damaged tissue showed higher expression of GFAP protein in the astrocytes of the treatment group than in the other two groups and the chitosan group compared with the negative control group. A controlled drug delivery system containing selenium nanoparticles seems to play a role in the protection of nerve cells through its anti-inflammatory effect.












Similar content being viewed by others
References
Yip PK, Malaspina A (2012) Spinal cord trauma and the molecular point of no return. Mol Neurodegener 7(1):6
Fehlings MG, Cadotte DW, Fehlings LN (2011) A series of systematic reviews on the treatment of acute spinal cord injury: a foundation for best medical practice. J Neurotrauma 28(8):1329–1333
Tator CH (1995) Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol 5(4):407–413
Profyris C, Cheema SS, Zang D, Azari MF, Boyle K, Petratos S (2004) Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis 15(3):415–436
Brown KM, Arthur J (2001) Selenium, selenoproteins and human health: a review. Public Health Nutr 4(2b):593–599
Javdani M, Habibi A, Shirian S, Kojouri GA, Hosseini F (2019) Effect of selenium nanoparticle supplementation on tissue inflammation, blood cell count, and IGF-1 levels in spinal cord injury-induced rats. Biol Trace Elem Res 187(1):202–211
Rao S, Lin Y, Du Y, He L, Huang G, Chen B, Chen T (2019) Designing multifunctionalized selenium nanoparticles to reverse oxidative stress-induced spinal cord injury by attenuating ROS overproduction and mitochondria dysfunction. J Mater Chem:B16. https://doi.org/10.1039/C8TB02520G
Heller RA, Seelig J, Bock T, Haubruck P, Grützner PA, Schomburg L, Moghaddam A, Biglari B (2019) Relation of selenium status to neuro-regeneration after traumatic spinal cord injury. J Trace Elem Med Biol 51:141–149
Chen M et al (2018) Efficient overcoming of blood–brain barrier by functionalized selenium nanoparticles to treat glioma. Adv Ther 1(8):1800074
Bhattarai N, Gunn J, Zhang M (2010) Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev 62(1):83–99
Ren YJ, Zhang H, Huang H, Wang XM, Zhou ZY, Cui FZ, An YH (2009) In vitro behavior of neural stem cells in response to different chemical functional groups. Biomaterials 30(6):1036–1044
Yang Y, Liu M, Gu Y, Lin S, Ding F, Gu X (2009) Effect of chitooligosaccharide on neuronal differentiation of PC-12 cells. Cell Biol Int 33(3):352–356
Gnavi S, Barwig C, Freier T, Haastert-Talini K, Grothe C, Geuna S (2013) The use of chitosan-based scaffolds to enhance regeneration in the nervous system. Int Rev Neurobiol 109:1–62
Hoare TR, Kohane DS (2008) Hydrogels in drug delivery: progress and challenges. Polymer 49(8):1993–2007
Natarajan JV, Nugraha C, Ng XW, Venkatraman S (2014) Sustained-release from nanocarriers: a review. J Control Release 193:122–138
Furuike T, Komoto D, Hashimoto H, Tamura H (2017) Preparation of chitosan hydrogel and its solubility in organic acids. Int J Biol Macromol 104:1620–1625
Verma P, Maheshwari SK (2018) Preparation of sliver and selenium nanoparticles and its characterization by dynamic light scattering and scanning electron microscopy. J Microsc Ultrastruct 6(4):182
Yoshizawa T, Kadekawa K, Tyagi P, Yoshikawa S, Takahashi R, Takahashi S, Yoshimura N (2015) Mechanisms inducing autonomic dysreflexia during urinary bladder distention in rats with spinal cord injury. Spinal Cord 53(3):190–194
Holmes GM, Blanke EN (2019) Gastrointestinal dysfunction after spinal cord injury. Exp Neurol 320:113009
Tykhomyrov А, Pavlova A, Nedzvetsky V (2016) Glial fibrillary acidic protein (GFAP): on the 45th anniversary of its discovery. Neurophysiology 48(1):54–71
Tian R, Wu X, Hagemann TL, Sosunov AA, Messing A, McKhann M, Goldman JE (2010) Alexander disease mutant glial fibrillary acidic protein compromises glutamate transport in astrocytes. J Neuropathol Exp Neurol 69(4):335–345
Hol EM, Pekny M (2015) Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol 32:121–113
Tardy M, Fages C, Le Prince G, Rolland B, Nunez J (1990) Regulation of the glial fibrillary acidic protein (GFAP) and of its encoding mRNA in the developing brain and in cultured astrocytes. In: Lauder JM, Privat A, Giacobini E, Timiras PS, Vernadakis A (eds) Molecular aspects of development and aging of the nervous system. advances in experimental medicine and biology, vol 265. Springer, Boston, pp 41–52
McCall M, Gregg RG, Behringer RR, Brenner M, Delaney CL, Galbreath EJ, Zhang CL, Pearce RA, Chiu SY, Messing A (1996) Targeted deletion in astrocyte intermediate filament (Gfap) alters neuronal physiology. Proc Natl Acad Sci 93(13):6361–6366
Zhang N, Yin Y, Xu SJ, Wu YP, Chen WS (2012) Inflammation & apoptosis in spinal cord injury. Indian J Med Res 135(3):287–296
Bach LA (2004) The insulin-like growth factor system: towards clinical applications. Clin Biochem Rev 25(3):155–164
Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J (2008) Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci 28(38):9330–9341
Schnell L, Fearn S, Klassen H, Schwab ME, Perry VH (1999) Acute inflammatory responses to mechanical lesions in the CNS: differences between brain and spinal cord. Eur J Neurosci 11(10):3648–3658
Sun X, Jones ZB, Chen XM, Zhou L, So KF, Ren Y (2016) Multiple organ dysfunction and systemic inflammation after spinal cord injury: a complex relationship. J Neuroinflammation 13(1):260
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24(9):2143–2155
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35
Nguyen T, Mao Y, Sutherland T, Gorrie CA (2017) Neural progenitor cells but not astrocytes respond distally to thoracic spinal cord injury in rat models. Neural Regen Res 12(11):1885–1894
Agulhon C, Petravicz J, McMullen AB, Sweger EJ, Minton SK, Taves SR, Casper KB, Fiacco TA, McCarthy KD (2008) What is the role of astrocyte calcium in neurophysiology? Neuron 59(6):932–946
Ricciotti E, FitzGerald GA (2011) Prostaglandins and Inflammation. Arterioscler Thromb Vasc Biol 31(5):986–1000
Yeo JE, Kang SK (2007) Selenium effectively inhibits ROS-mediated apoptotic neural precursor cell death in vitro and in vivo in traumatic brain injury. Biochim Biophys Acta 1772(11-12):1199–1210
Ruppert K, Olson S, Cox C (2019) Spinal cord injury. In: a roadmap to non-hematopoietic stem cell-based therapeutics. Elsevier, pp 321–343
Tinggi U (2003) Essentiality and toxicity of selenium and its status in Australia: a review. Toxicol Lett 137(1-2):103–110
Kizilay Z, Erken HA, Aktas S, Ersoy N, Abas EI, Topcu A, Yenisey Ç, Ismailoglu O (2017) Effects of selenium on axon and myelin healing in an experimental sciatic nerve injury model. J Ist Faculty Med 80(4):138–145
Chedly J, Soares S, Montembault A, von Boxberg Y, Veron-Ravaille M, Mouffle C, Benassy MN, Taxi J, David L, Nothias F (2017) Physical chitosan microhydrogels as scaffolds for spinal cord injury restoration and axon regeneration. Biomaterials 138:91–107
Author information
Authors and Affiliations
Contributions
Moosa Javdani, as a manager of the current investigation and corresponding author, designed and performed experiments and also wrote the paper. Roya Ghorbani, as a co-worker, was involved in performing the work. Mohammad Hashemnia, as a co-worker and histologist, performed the laboratory part of this study.
Corresponding author
Ethics declarations
All the investigation procedures used in the current study were reviewed and approved by the Research Council of the Faculty of Veterinary Medicine of Shahrekord University (170-1204).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Javdani, M., Ghorbani, R. & Hashemnia, M. Histopathological Evaluation of Spinal Cord with Experimental Traumatic Injury Following Implantation of a Controlled Released Drug Delivery System of Chitosan Hydrogel Loaded with Selenium Nanoparticle. Biol Trace Elem Res 199, 2677–2686 (2021). https://doi.org/10.1007/s12011-020-02395-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02395-2