Abstract
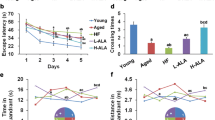
Alzheimer’s disease is characterized by the aggregation of amyloid-beta (Aβ) peptide into plaques and neurofibrillary tangles. Aβ peptide is generated by the cleavage of the β-amyloid precursor protein (APP) by β- and γ-secretase. The present study was conducted to investigate the effects of fish oil (or eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)), selenium, and zinc on learning and memory impairment in an aging mouse model and on APP. We performed the Morris water maze and platform recorder tests on male Kunming mice (10/group) grouped as control and d-galactose-induced aging model mice treated with vehicle, fish oil, fish oil + selenium, fish oil + selenium + zinc, and positive control (red ginseng extract). Fish oil + zinc + selenium for 7 weeks significantly improved learning and memory impairments in aging model animals in the Morris water maze and platform recorder tests, as evidenced by shortened incubation periods and number of errors. In vitro analysis of Aβ1–40 content in APP695-transfected CHO cells revealed a decrease after treatment with EPA, DHA, and their combinations with selenium or selenium and zinc. Assaying β- and γ-secretase activities revealed a decrease in PC12 cells and mouse serum as well as a decrease in β-site APP-cleaving enzyme 1 and presenilin 1 protein levels in the PC12 cells and mouse serum. Taken together, our results show that fish oil combined with selenium and zinc inhibited APP processing and alleviated learning and memory impairment in a mouse model of aging.






Similar content being viewed by others
References
Philippens IH, Ormel PR, Baarends G, Johansson M, Remarque EJ, Doverskog M (2017) Acceleration of amyloidosis by inflammation in the amyloid-beta marmoset monkey model of Alzheimer’s disease. J Alzheimers Dis 55(1):101–113. https://doi.org/10.3233/JAD-160673
Jakob-Roetne R, Jacobsen H (2009) Alzheimer’s disease: from pathology to therapeutic approaches. Angew Chem Int Ed Eng 48(17):3030–3059. https://doi.org/10.1002/anie.200802808
Hernandez-Rapp J, Rainone S, Goupil C, Dorval V, Smith PY, Saint-Pierre M, Vallee M, Planel E, Droit A, Calon F, Cicchetti F, Hebert SS (2016) microRNA-132/212 deficiency enhances Abeta production and senile plaque deposition in Alzheimer’s disease triple transgenic mice. Sci Rep 6:30953. https://doi.org/10.1038/srep30953
Majd S, Power J, Majd Z (2019) Alzheimer’s disease and cancer: when two monsters cannot be together. Front Neurosci 13:155. https://doi.org/10.3389/fnins.2019.00155
Wang Y, Liu C, Wang H, Jiang Y, Wang P, Shang H (2019) Systematic review of basic research on Alzheimer’s disease with Shen Zhi Ling oral liquid. Evid Based Complement Alternat Med 2019:8216714–8216710. https://doi.org/10.1155/2019/8216714
Elbaum-Garfinkle S (2019) Matter over mind: liquid phase separation and neurodegeneration. J Biol Chem 294(18):7160–7168. https://doi.org/10.1074/jbc.REV118.001188
Gomes E, Shorter J (2019) The molecular language of membraneless organelles. J Biol Chem 294(18):7115–7127. https://doi.org/10.1074/jbc.TM118.001192
Dinkova-Kostova AT, Kostov RV, Kazantsev AG (2018) The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J 285(19):3576–3590. https://doi.org/10.1111/febs.14379
Raefsky SM, Furman R, Milne G, Pollock E, Axelsen P, Mattson MP, Shchepinov MS (2018) Deuterated polyunsaturated fatty acids reduce brain lipid peroxidation and hippocampal amyloid beta-peptide levels, without discernable behavioral effects in an APP/PS1 mutant transgenic mouse model of Alzheimer’s disease. Neurobiol Aging 66:165–176. https://doi.org/10.1016/j.neurobiolaging.2018.02.024
Tcw J, Goate AM (2017) Genetics of beta-amyloid precursor protein in Alzheimer’s disease. Cold Spring Harb Perspect Med 7(6). https://doi.org/10.1101/cshperspect.a024539
Fan YG, Guo T, Han XR, Liu JL, Cai YT, Xue H, Huang XS, Li YC, Wang ZY, Guo C (2019) Paricalcitol accelerates BACE1 lysosomal degradation and inhibits calpain-1 dependent neuronal loss in APP/PS1 transgenic mice. EBioMedicine 45:393–407. https://doi.org/10.1016/j.ebiom.2019.07.014
Ghosh AK, Osswald HL (2014) BACE1 (beta-secretase) inhibitors for the treatment of Alzheimer’s disease. Chem Soc Rev 43(19):6765–6813. https://doi.org/10.1039/c3cs60460h
Sun L, Zhou R, Yang G, Shi Y (2017) Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Abeta42 and Abeta40 peptides by gamma-secretase. Proc Natl Acad Sci U S A 114(4):E476–E485. https://doi.org/10.1073/pnas.1618657114
Shinohara M, Tachibana M, Kanekiyo T, Bu G (2017) Role of LRP1 in the pathogenesis of Alzheimer’s disease: evidence from clinical and preclinical studies. J Lipid Res 58(7):1267–1281. https://doi.org/10.1194/jlr.R075796
Xiang J, Zhang W, Cai XF, Cai M, Yu ZH, Yang F, Zhu W, Li XT, Wu T, Zhang JS, Cai DF (2019) DNA Aptamers targeting BACE1 reduce amyloid levels and rescue neuronal deficiency in cultured cells. Mol Ther Nucleic Acids 16:302–312. https://doi.org/10.1016/j.omtn.2019.02.025
Binyamin O, Nitzan K, Frid K, Ungar Y, Rosenmann H, Gabizon R (2019) Brain targeting of 9c,11t-conjugated linoleic acid, a natural calpain inhibitor, preserves memory and reduces Abeta and P25 accumulation in 5XFAD mice. Sci Rep 9(1):18437. https://doi.org/10.1038/s41598-019-54971-9
Echeverria F, Valenzuela R, Catalina Hernandez-Rodas M, Valenzuela A (2017) Docosahexaenoic acid (DHA), a fundamental fatty acid for the brain: new dietary sources. Prostaglandins Leukot Essent Fat Acids 124:1–10. https://doi.org/10.1016/j.plefa.2017.08.001
Cosin-Tomas M, Senserrich J, Arumi-Planas M, Alquezar C, Pallas M, Martin-Requero A, Sunol C, Kaliman P, Sanfeliu C (2019) Role of resveratrol and selenium on oxidative stress and expression of antioxidant and anti-aging genes in immortalized lymphocytes from Alzheimer’s disease patients. Nutrients 11(8). https://doi.org/10.3390/nu11081764
Bitanihirwe BK, Cunningham MG (2009) Zinc: the brain’s dark horse. Synapse 63(11):1029–1049. https://doi.org/10.1002/syn.20683
Alehagen U, Johansson P, Bjornstedt M, Rosen A, Dahlstrom U (2013) Cardiovascular mortality and N-terminal-proBNP reduced after combined selenium and coenzyme Q10 supplementation: a 5-year prospective randomized double-blind placebo-controlled trial among elderly Swedish citizens. Int J Cardiol 167(5):1860–1866. https://doi.org/10.1016/j.ijcard.2012.04.156
Zhu JD, Wang JJ, Zhang XH, Yu Y, Kang ZS (2018) Panax ginseng extract attenuates neuronal injury and cognitive deficits in rats with vascular dementia induced by chronic cerebral hypoperfusion. Neural Regen Res 13(4):664–672. https://doi.org/10.4103/1673-5374.230292
Barnhart CD, Yang D, Lein PJ (2015) Using the Morris water maze to assess spatial learning and memory in weanling mice. PLoS One 10(4):e0124521. https://doi.org/10.1371/journal.pone.0124521
Seok H, Lee M, Shin E, Yun MR, Lee YH, Moon JH, Kim E, Lee PH, Lee BW, Kang ES, Lee HC, Cha BS (2019) Low-dose pioglitazone can ameliorate learning and memory impairment in a mouse model of dementia by increasing LRP1 expression in the hippocampus. Sci Rep 9(1):4414. https://doi.org/10.1038/s41598-019-40736-x
Zhou X, Wang L, Xiao W, Su Z, Zheng C, Zhang Z, Wang Y, Xu B, Yang X, Hoi MPM (2019) Memantine improves cognitive function and alters hippocampal and cortical proteome in triple transgenic mouse model of Alzheimer’s disease. Exp Neurobiol 28(3):390–403. https://doi.org/10.5607/en.2019.28.3.390
Li GZ, Liu F, Xu C, Li JY, Xu YJ (2018) Selenium and zinc against Abeta25-35-induced cytotoxicity and tau phosphorylation in PC12 cells and inhibits gamma-cleavage of APP. Biol Trace Elem Res 184(2):442–449. https://doi.org/10.1007/s12011-017-1162-4
Jia D, Heng LJ, Yang RH, Gao GD (2014) Fish oil improves learning impairments of diabetic rats by blocking PI3K/AKT/nuclear factor-kappaB-mediated inflammatory pathways. Neuroscience 258:228–237. https://doi.org/10.1016/j.neuroscience.2013.11.016
Yang RH, Wang F, Hou XH, Cao ZP, Wang B, Xu XN, Hu SJ (2012) Dietary omega-3 polyunsaturated fatty acids improves learning performance of diabetic rats by regulating the neuron excitability. Neuroscience 212:93–103. https://doi.org/10.1016/j.neuroscience.2012.04.005
Yousef M, Kavraal S, Artis AS, Suer C (2019) Effects of chronic and acute lithium treatment on the long-term potentiation and spatial memory in adult rats. Clin Psychopharmacol Neurosci 17(2):233–243. https://doi.org/10.9758/cpn.2019.17.2.233
Li JG, Chu J, Barrero C, Merali S, Pratico D (2014) Homocysteine exacerbates beta-amyloid pathology, tau pathology, and cognitive deficit in a mouse model of Alzheimer disease with plaques and tangles. Ann Neurol 75(6):851–863. https://doi.org/10.1002/ana.24145
Fang F, Yu Q, Arancio O, Chen D, Gore SS, Yan SS, Yan SF (2018) RAGE mediates Abeta accumulation in a mouse model of Alzheimer’s disease via modulation of beta- and gamma-secretase activity. Hum Mol Genet 27(6):1002–1014. https://doi.org/10.1093/hmg/ddy017
Van der Jeugd A, Parra-Damas A, Baeta-Corral R, Soto-Faguas CM, Ahmed T, LaFerla FM, Gimenez-Llort L, D’Hooge R, Saura CA (2018) Reversal of memory and neuropsychiatric symptoms and reduced tau pathology by selenium in 3xTg-AD mice. Sci Rep 8(1):6431. https://doi.org/10.1038/s41598-018-24741-0
Moncayo R, Ortner K (2015) Multifactorial determinants of cognition - thyroid function is not the only one. BBA Clin 3:289–298. https://doi.org/10.1016/j.bbacli.2015.04.002
Bazazzadegan N, Dehghan Shasaltaneh M, Saliminejad K, Kamali K, Banan M, Khorram Khorshid HR (2017) Effects of herbal compound (IMOD) on behavior and expression of Alzheimer’s disease related genes in streptozotocin-rat model of sporadic Alzheimer’s disease. Adv Pharm Bull 7(3):491–494. https://doi.org/10.15171/apb.2017.060
Wong E, Liao GP, Chang JC, Xu P, Li YM, Greengard P (2019) GSAP modulates gamma-secretase specificity by inducing conformational change in PS1. Proc Natl Acad Sci U S A 116(13):6385–6390. https://doi.org/10.1073/pnas.1820160116
Chalatsa I, Arvanitis DA, Koulakiotis NS, Giagini A, Skaltsounis AL, Papadopoulou-Daifoti Z, Tsarbopoulos A, Sanoudou D (2019) The Crocus sativus compounds trans-crocin 4 and trans-crocetin modulate the amyloidogenic pathway and tau misprocessing in Alzheimer disease neuronal cell culture models. Front Neurosci 13:249. https://doi.org/10.3389/fnins.2019.00249
Wang Z, Xu Q, Cai F, Liu X, Wu Y, Song W (2019) BACE2, a conditional beta-secretase, contributes to Alzheimer’s disease pathogenesis. JCI Insight 4(1). https://doi.org/10.1172/jci.insight.123431
Koivisto H, Grimm MO, Rothhaar TL, Berkecz R, Lutjohann DD, Giniatullina R, Takalo M, Miettinen PO, Lahtinen HM, Giniatullin R, Penke B, Janaky T, Broersen LM, Hartmann T, Tanila H (2014) Special lipid-based diets alleviate cognitive deficits in the APPswe/PS1dE9 transgenic mouse model of Alzheimer’s disease independent of brain amyloid deposition. J Nutr Biochem 25(2):157–169. https://doi.org/10.1016/j.jnutbio.2013.09.015
Dekker AD, Fortea J, Blesa R, De Deyn PP (2017) Cerebrospinal fluid biomarkers for Alzheimer’s disease in Down syndrome. Alzheimers Dement (Amst) 8:1–10. https://doi.org/10.1016/j.dadm.2017.02.006
Funding
This work was financially supported by the Research Foundation of TCM Science and Technology Project of Jilin Province (2018-135), Jilin Provincial Education Department (JJKH20200532KJ), National Science Foundation of China (81660142), and China Scholarship Council (2016-3192).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experimental design was approved by the Council on Animal Care Committee, Medical College of Yanbian University, China.
Conflict of Interest
The authors declare that they have no conflict of interest
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fu, Cx., Dai, L., Yuan, Xy. et al. Effects of Fish Oil Combined with Selenium and Zinc on Learning and Memory Impairment in Aging Mice and Amyloid Precursor Protein Processing. Biol Trace Elem Res 199, 1855–1863 (2021). https://doi.org/10.1007/s12011-020-02280-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02280-y