Abstract
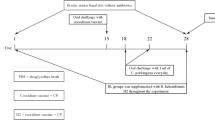
This study was carried out to investigate the effects of selenium-enriched probiotics (SP) supplementation on growth performance, oocysts shedding, intestinal lesions and antioxidant capacities, and mRNA gene expression of local Chinese yellow male chickens infected with Eimeria tenella. One-day-old 270 chickens were randomly assigned into five groups, each consisting of three replicates with 18 chickens per replicate. Chickens in the negative and positive controls (NC, PC, respectively) received basal diets only (0.11 mg Se/kg), whereas the other groups were supplied basal diets with probiotics and designated as (P, 0.11 mg Se/kg), sodium selenite (SS, 0.41 mg Se/kg), and (SP, 0.41 mg Se/kg) groups. At 21 days of age, except the NC group, all other groups were infected by oral gavage with 1.5 × 104 sporulated E. tenella oocysts per chicken. Three chickens were randomly selected from each group for serum, liver, and cecal specimen collection. The results showed that P, SS, and SP had significant increase weight gain and feed intake. Additionally, these groups showed higher activities of serum superoxide dismutase (SOD) and glutathione peroxidase-1 (GPx1) compared to the PC group, whereas feed conversion ratio (FCR), serum catalase (CAT) activity, and malondialdehyde (MDA) content remained lower. Moreover, P, SS, and SP groups had lower oocyst shedding and cecal lesion scores. Significant upregulation of the glutathione peroxidase-1 (GPx1), glutathione peroxidase-4 (GPx4), Selenium W (SelW), and interferon gamma (IFN-γ) mRNA expression were detected in the SP group, which was then followed by SS when compared to the P group, whereas mRNA expression down-regulated in the PC group compared to NC, P, SS, and SP. In the NC and P groups, there were no significant differences in mRNA expression, except that IFN-γ mRNA level upregulated in P. We concluded that selenium-enriched probiotic supplementation has profound effects in enhancing the growth performance, antioxidant capacities, mRNA gene expression, reduced of oocysts shedding, and the cecal lesion scores of chickens and do provide protection against E. tenella.


Similar content being viewed by others
References
Lee H-A, Hong S, Chung Y, Kim O (2011) Sensitive and specific identification by polymerase chain reaction of Eimeria tenella and Eimeria maxima, important protozoan pathogens in laboratory avian facilities. Lab Anim Res 27:255–258. https://doi.org/10.5625/lar.2011.27.3.255
Rathinam T, Gadde U, Chapman HD (2015) Molecular detection of field isolates of Turkey Eimeria by polymerase chain reaction amplification of the cytochrome c oxidase I gene. https://doi.org/10.1007/s00436-015-4546-4
Saif YM, Faldy AM, Calnek BW et al (2003) Diseases of poultry, 11th edn
Alnassan AA, Thabet A, Daugschies A, Bangoura B (2015) In vitro efficacy of allicin on chicken Eimeria tenella sporozoites:4–6. https://doi.org/10.1007/s00436-015-4637-2
Min W, Kim WH, Lillehoj EP, Lillehoj HS (2013) Recent progress in host immunity to avian coccidiosis : IL-17 family cytokines as sentinels of the intestinal mucosa. Dev Comp Immunol 41:418–428. https://doi.org/10.1016/j.dci.2013.04.003
Dalloul RA, Lillehoj HS, Lee JS, Lee SH, Chung KS (2006) Immunopotentiating effect of a Fomitella fraxinea-derived lectin on chicken immunity and resistance to coccidiosis. Poult Sci 85:446–451. https://doi.org/10.1093/ps/85.3.446
Hoan TD, Thao DT, Gadahi JA, Song X, Xu L, Yan R, Li X (2014) Analysis of humoral immune response and cytokines in chickens vaccinated with Eimeria brunetti apical membrane antigen-1 (EbAMA1) DNA vaccine. Exp Parasitol 144:65–72. https://doi.org/10.1016/j.exppara.2014.04.015
Giannenas I, Tsalie E, Triantafillou E, Hessenberger S, Teichmann K, Mohnl M, Tontis D (2014) Assessment of probiotics supplementation via feed or water on the growth performance, intestinal morphology and microflora of chickens after experimental infection with Eimeria acervulina, Eimeria maxima and Eimeria tenella. Avian Pathol 43:209–216. https://doi.org/10.1080/03079457.2014.899430
Bun SD, Guo YM, Guo FC, Ji FJ, Cao H (2011) Influence of organic zinc supplementation on the antioxidant status and immune responses of broilers challenged with Eimeria tenella. Poult Sci 90:1220–1226. https://doi.org/10.3382/ps.2010-01308
Dalloul RA, Lillehoja HS (2016) Invited minireview: recent advances in immunomodulation and vaccination strategies against coccidiosis. In: Dalloul RA, Lillehoj HS. Published by: American Association of Avian Pathologists Stable. 49:1–8. URL: http://www.jstor.org/stable
Bordoni A, Danesi F, Malaguti M et al (2008) Dietary selenium for the counteraction of oxidative damage: fortified foods or supplements? Br J Nutr 99:191–197. https://doi.org/10.1017/S0007114507793911
Liu CP, Fu J, Lin SL, Wang XS, Li S (2014) Effects of dietary selenium deficiency on mRNA levels of twenty-one selenoprotein genes in the liver of layer chicken. Biol Trace Elem Res 159:192–198
Celi P, Cowieson AJ, Fru-Nji F et al (2017) Gastrointestinal functionality in animal nutrition and health: new opportunities for sustainable animal production. Anim Feed Sci Technol 234:88–100
Colnago GL, Jensen LS, Long PL (1984) Effect of selenium and vitamin E on the development of immunity to coccidiosis in chickens. Poult Sci 63:1136–1143
Yang J, Huang K, Qin S, Wu X, Zhao Z, Chen F (2009) Antibacterial action of selenium-enriched probiotics against pathogenic Escherichia coli. Dig Dis Sci 54:246–254
Farnell MB, Donoghue AM, Santos FSDL, et al (2006) Immunology, health, and disease upregulation of oxidative burst and degranulation in chicken heterophils stimulated with probiotic bacteria. 1900–1906
Lessard M, Dupuis M, Gagnon N et al (2014) Modulates development of porcine mucosal immunity and reduces intestinal bacterial boulardii modulates development of porcine mucosal immunity and reduces intestinal bacterial translocation:922–934. https://doi.org/10.2527/jas.2008-0919
Zulkifli I, Abdullah N, Azrin NM, Ho YW (2000) Growth performance and immune response of two commercial broiler strains fed diets containing lactobacillus cultures and oxytetracycline under heat stress conditions. Br Poult Sci 41:593–597. https://doi.org/10.1080/713654979
Dalloul RA, Lillehoj HS, Lee J, et al (2005) Immunopotentiating effect of a Fomitella fraxinea -derived Lectin on chicken immunity and resistance to Coccidiosis. 446–451
Pan C, Zhao Y, Liao SF, Chen F, Qin S, Wu X, Zhou H, Huang K (2011) Effect of selenium-enriched probiotics on laying performance, egg quality, egg selenium content, and egg glutathione peroxidase activity. J Agric Food Chem 59:11424–11431
Gan F, Ren F, Chen X, et al (2013) Effects of selenium-enriched probiotics on heat shock protein mRNA levels in piglet under heat stress conditions
Hodgson JN (1970) Coccidiosis: oocyst counting technique for coccidiostat evaluation. Exp Parasitol 28:99–102
Ave C, Washington NW (2013) Nutrient requirements of chickens and turkeys. 1994:1–11
Khan AZ, Kumbhar S, Hamid M et al (2016) Effects of selenium-enriched probiotics on heart lesions by influencing the mRNA expressions of selenoproteins and heat shock proteins in heat stressed broiler chickens. Pak Vet J:36
Foreyt WJ (2001) Parasites of cats. In: Vet Parasitol ref manual, 5th edn. Iowa State Univ Press, Ames, pp 50–61
Patel JB, Cockerill RF, Bradford AP, Eliopoulos MG, Hindler AJ, Jenkins GS, Lewis SJ, Limbago B, Miller AL, Nicolau PD, Pwell M, Swenson MJ, Traczewski MM, Turnidge JD WPMZLB (2015) M07-A10: methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—10th Edition. CLSI (Clinical Lab Stand Institute) 35. https://doi.org/10.1007/s00259-009-1334-3
Bancroft NH, Designed By-Cunningham F (1996) Implementing SAP r/3. Prentice Hall PTR
Johnson J, Reid WM (1970) Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol 28:30–36. https://doi.org/10.1016/0014-4894(70)90063-9
Adamu M, Boonkaewwan C, Gongruttananun N, Vongpakorn M (2013) Hematological, biochemical and histopathological changes caused by Coccidiosis in chickens. Kasetsart J (Nat Sci) 47:238–246
Ran L, Wu X, Shen X, Zhang K, Ren F, Huang K (2010) Effects of selenium form on blood and milk selenium concentrations, milk component and milk fatty acid composition in dairy cows. J Sci Food Agric 90:2214–2219. https://doi.org/10.1002/jsfa.4073
Aebi A (1984) Catalase in vitro. Methods Enzymol 105:121–126
Panchenko LF, Brusov OS, Gerasimov AM, Loktaeva TD (1975) Intramitochondrial localization and release of rat liver superoxide dismutase. FEBS Lett 55:84–87
Lawrence RA (2012) Reprint of “glutathione peroxidase activity in selenium-deficient rat liver.”. Biochem Biophys Res Commun 425:503–509. https://doi.org/10.1016/j.bbrc.2012.08.016
Placer ZA, Cushman LL, Johnson BC (1966) Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem 16:359–364
Liang Y, Lin SL, Wang CW, Yao HD, Zhang ZW, Xu SW (2014) Effect of selenium on selenoprotein expression in the adipose tissue of chickens. Biol Trace Elem Res 160:41–48. https://doi.org/10.1007/s12011-014-0024-6
Wu Q, Yao HD, Tan SR et al (2014) Possible correlation of Selenoprotein W with inflammation factors in chicken skeletal muscles. Biol Trace Elem Res 161:167–172. https://doi.org/10.1007/s12011-014-0092-7
Laurent F, Mancassola R, Lacroix S, Menezes R, Naciri M (2001) Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect Immun 69:2527–2534
Yu J, Bao E, Yan J, Lei L (2008) Expression and localization of Hsps in the heart and blood vessel of heat-stressed broilers. Cell Stress Chaperones 13:327–335. https://doi.org/10.1007/s12192-008-0031-7
Bernardini C, Zannoni A, Turba ME, Bacci ML, Forni M, Mesirca P, Remondini D, Castellani G, Bersani F (2007) Effects of 50 Hz sinusoidal magnetic fields on Hsp27, Hsp70, Hsp90 expression in porcine aortic endothelial cells (PAEC). Bioelectromagnetics 28:231–237. https://doi.org/10.1002/bem.20299
Giannenas I, Papadopoulos E, Tsalie E et al (2012) Assessment of dietary supplementation with probiotics on performance, intestinal morphology and microflora of chickens infected with Eimeria tenella. Vet Parasitol 188:31–40. https://doi.org/10.1016/j.vetpar.2012.02.017
Wallace RJ, Oleszek W, Franz C, Hahn I, Baser KH, Mathe A, Teichmann K (2010) Dietary plant bioactives for poultry health and productivity. Br Poult Sci 51:461–487. https://doi.org/10.1080/00071668.2010.506908
M’Sadeq SA, Wu S, Swick RA, Choct M (2015) Towards the control of necrotic enteritis in broiler chickens with in-feed antibiotics phasing-out worldwide. Anim Nutr 1:1–11. https://doi.org/10.1016/j.aninu.2015.02.004
Singh VS, Palod J, Vatsya S, Shukla SK (2013) Effect of herbal vitamin E-selenium with organic chromium on growth , haematological and parasitological parameters of broiler chicken during mixed Eimeria infection. 1:25–30
Lee KW, Lee SH, Lillehoj HS, Li GX, Jang SI, Babu US, Park MS, Kim DK, Lillehoj EP, Neumann AP, Rehberger TG, Siragusa GR (2010) Effects of direct-fed microbials on growth performance, gut morphometry, and immune characteristics in broiler chickens. Poult Sci 89:203–216. https://doi.org/10.3382/ps.2009-00418
Nayebpor M, Farhomand P, Hashemi A (2007) Effects of different levels of direct fed microbial (Primalac) on growth performance and humoral immune response in broiler chickens. J Anim Vet Adv 6:1308–1313
Allen PC (1997) Production of free radical species during Eimeria maxima infections in chickens. Poult Sci 76:814–821. https://doi.org/10.1093/ps/76.6.814
Georgieva NV, Koinarski V, Gadjeva V (2006) Antioxidant status during the course of Eimeria tenella infection in broiler chickens. Vet J 172:488–492. https://doi.org/10.1016/j.tvjl.2005.07.016
Wang ML, Suo X, Gu JH, Zhang WW, Fang Q, Wang X (2008) Influence of grape seed proanthocyanidin extract in broiler chickens: effect on chicken coccidiosis and antioxidant status. Poult Sci 87:2273–2280. https://doi.org/10.3382/ps.2008-00077
Pophaly SD, Poonam SP et al (2014) Selenium enrichment of lactic acid bacteria and bifidobacteria: a functional food perspective. Trends Food Sci Technol 39:135–145. https://doi.org/10.1016/j.tifs.2014.07.006
Balogh K, Kovács-Weber M, Erdélyi M, Mézes M (2007) Investigation of lipid peroxide and glutathione redox status of chicken concerning on high dietary selenium intake. Acta Biol Hung 58:269–279. https://doi.org/10.1556/ABiol.58.2007.3.3
Koinarski V, Sotirov LK, Denev S (2012) Serum lysozyme concentrations in broilers treated with Sel-Plex® and sodium selenite and infected with Eimeria tenella. Turk J Vet Anim Sci 36:433–437. https://doi.org/10.3906/vet-0711-38
Medici M, Vinderola CG, Perdigón G (2004) Gut mucosal immunomodulation by probiotic fresh cheese. Int Dairy J 14:611–618. https://doi.org/10.1016/j.idairyj.2003.10.011
Georgieva NV, Gabrashanska M, Koinarski V, Ermidou-Pollet S (2011) Antioxidant status in Eimeria acervulina infected chickens after dietary selenium treatment. Trace Elem Electrolytes 28:42–48. https://doi.org/10.5414/TEP28042
Yu D, Zhang Z, Yao H, Li S, Xu SW (2015) The role of selenoprotein W in inflammatory injury in chicken immune tissues and cultured splenic lymphocyte. BioMetals 28:75–87. https://doi.org/10.1007/s10534-014-9804-x
Reeves MA, Hoffmann PR (2009) The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci 66:2457–2478. https://doi.org/10.1007/s00018-009-0032-4
Pagmantidis V, Bermano G, Villette S, Broom I, Arthur J, Hesketh J (2005) Effects of se-depletion on glutathione peroxidase and selenoprotein W gene expression in the colon. FEBS Lett 579:792–796. https://doi.org/10.1016/j.febslet.2004.12.042
Yu D, Li JL, Zhang JL et al (2011) Effects of dietary selenium on selenoprotein W gene expression in the chicken immune organs. Biol Trace Elem Res 144:678–687. https://doi.org/10.1007/s12011-011-9062-5
Yuan D, Zhan XA, Wang YX (2012) Effect of selenium sources on the expression of cellular glutathione peroxidase and cytoplasmic thioredoxin reductase in the liver and kidney of broiler breeders and their offspring. Poult Sci 91:936–942. https://doi.org/10.3382/ps.2011-01921
Gan F, Chen X, Liao SF, Lv C, Ren F, Ye G, Pan C, Huang D, Shi J, Shi X, Zhou H, Huang K (2014) Selenium-enriched probiotics improve antioxidant status, immune function, and selenoprotein gene expression of piglets raised under high ambient temperature. J Agric Food Chem 62:4502–4508. https://doi.org/10.1021/jf501065d
Hill KE, Reid Lyons P, Burk RF (1992) Differential regulation of rat liver selenoprotein mRNAs in selenium deficiency. Biochem Biophys Res Commun 185:260–263. https://doi.org/10.1016/S0006-291X(05)80984-2
Sunde RA, Hadley KB (2010) Phospholipid hydroperoxide glutathione peroxidase (Gpx4) is highly regulated in male turkey poults and can be used to determine dietary selenium requirements. Exp Biol Med 235:23–31
Lee SH, Lillehoj HS, Jang SI, Lee KW, Bravo D, Lillehoj EP (2011) Effects of dietary supplementation with phytonutrients on vaccine-stimulated immunity against infection with Eimeria tenella. Vet Parasitol 181:97–105. https://doi.org/10.1016/j.vetpar.2011.05.003
Hong YH, Lillehoj HS, Lillehoj EP, Lee SH (2006) Changes in immune-related gene expression and intestinal lymphocyte subpopulations following Eimeria maxima infection of chickens. Vet Immunol Immunopathol 114:259–272. https://doi.org/10.1016/j.vetimm.2006.08.006
Xu S, Lee SH, Lillehoj HS et al (2015) Effects of dietary selenium on host response to necrotic enteritis in young broilers. Res Vet Sci 98:66–73. https://doi.org/10.1016/j.rvsc.2014.12.004
Choi KD, Lillehoj HS, Song KD, Han JY (1999) Molecular and functional characterization of chicken IL-15. Dev Comp Immunol 23:165–177. https://doi.org/10.1016/S0145-305X(98)00046-9
Dalloul RA, Lillehoj HS, Tamim NM et al (2005) Induction of local protective immunity to Eimeria acervulina by a Lactobacillus-based probiotic. Comp Immunol Microbiol Infect Dis 28:351–361. https://doi.org/10.1016/j.cimid.2005.09.001
Briens M, Mercier Y, Rouffineau F, Vacchina V, Geraert PA (2013) Comparative study of a new organic selenium source v. seleno-yeast and mineral selenium sources on muscle selenium enrichment and selenium digestibility in broiler chickens. Br J Nutr 110:617–624. https://doi.org/10.1017/S0007114512005545
Funding
The first author acknowledges the China scholarship council for the full grant of scholarship that covers all expenses during the study period. Authors would like to thank the National Natural Science Foundation of China (31272627, 31472253) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (Jiangsu, China) for funding this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mengistu, B.M., Bitsue, H.K. & Huang, K. The Effects of Selenium-Enriched Probiotics on Growth Performance, Oocysts Shedding, Intestinal Cecal Lesion Scores, Antioxidant Capacity, and mRNA Gene Expression in Chickens Infected with Eimeria tenella. Biol Trace Elem Res 199, 278–291 (2021). https://doi.org/10.1007/s12011-020-02118-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02118-7