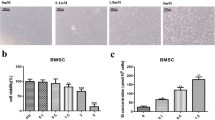
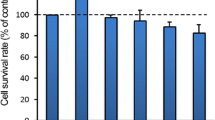
Abstract
Silicon-doped materials have been widely used in bone regeneration research; however, a consensus on the safety range of silicon ions has not been reached and its toxicity mechanism remains to be further elucidated. This study aims to explore whether high level of sodium metasilicate can induce toxicity effect in human umbilical vein endothelial cells (HUVEC) and the role of autophagy and apoptosis in its toxic mechanism. HUVEC was treated with different level of high silicon and then investigated with respect to morphologic change, cell viability, immunofluorescence, the level of autophagy, and apoptosis-related protein. Moreover, bafilomycin A1 (Baf A1) was applied to detect whether autophagic flux is disrupted, and 3-methyladenine (3-MA, an autophagy inhibitor) was used to determine the relationship between autophagy and apoptosis. Results demonstrated that high-level silicon induced cell viability to decrease; LC3-II, p62, and apoptosis-related proteins were up-regulated after exposure to high-dose silicon (sodium metasilicate concentration more than 1 mM). There is no significant difference in LC3-II and p62 between Baf A1 and sodium metasilicate-exposed group. Besides, 3-MA further increased the apoptotic rate by inhibiting autophagy after high silicon exposure. Collectively, high concentration of silicon can impair autophagy and induce apoptosis in human umbilical vein endothelial cells, and autophagy may play a protective role in HUVEC apoptosis. Furthermore, silicon concentration used in HUVEC should not be more than 1 mM.





Similar content being viewed by others
Abbreviations
- HUVEC:
-
Human umbilical vein endothelial cell
- CCK-8:
-
Cell Counting Kit-8
- BAX:
-
Bcl-2 associated X protein
- PARP:
-
Poly ADP-ribose polymerase
- LC3:
-
Light chain 3
- 3-MA:
-
3-Methyladenine
- Baf A1:
-
Bafilomycin A1
- ICP-AES:
-
Inductively coupled plasma atomic emission spectrometry
- DAPI:
-
4′,6-Diamidino-2-phenylindole
References
Carlisle EM (1970) Silicon: a possible factor in bone calcification. Science 167:279–280
Du M, Zhang J, Wang J, Hu L, Shan W (2012) Evaluation of silicon extract from leaves of phyllostachys edulis for topical anti-osteoporosis activity. In: World Automation Congress
Henstock JR, Canham LT, Anderson SI (2015) Silicon: the evolution of its use in biomaterials. Acta Biomater 11:17–26
Jugdaohsingh R (2007) Silicon and bone health. J Nutr Health Aging 11:99–110
Macdonald HM, Hardcastle AC, Jugdaohsingh R, Fraser WD, Reid DM, Powell JJ Dietary silicon interacts with oestrogen to influence bone health: evidence from the Aberdeen Prospective Osteoporosis Screening Study. Accessed (3)
Rodella LF, Bonazza V, Labanca M, Lonati C, Rezzani R (2014) A review of the effects of dietary silicon intake on bone homeostasis and regeneration. J Nutr Health Aging 18:820–826
KA H, PA R, N S and T B (2006) Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials 27:5014–5026
Dashnyam K, Elfiqi A, Buitrago JO, Perez RA, Knowles JC, Kim HW (2017) A mini review focused on the proangiogenic role of silicate ions released from silicon-containing biomaterials. J Tissue Eng 8 2041731417707339
Han P, Wu C, Xiao Y (2013) The effect of silicate ions on proliferation, osteogenic differentiation and cell signalling pathways (WNT and SHH) of bone marrow stromal cells. Biomaterialsence 1:379–392
Bennett C, Samikkannu M, Mohammed F, Dietrich WD, Rajguru SM, Prasad A (2018) Blood brain barrier (BBB)-disruption in intracortical silicon microelectrode implants. Biomaterials 164:1–10
Biran R, Martin DC, Tresco PA (2005) Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol 195:115–126
Thorburn A (2017) Autophagy and disease. J Biol Chem 293 jbcR117.810739
Marino G, Niso-Santano M, Baehrecke EH, Kroemer G (2014) Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol 15:81–94
Booth LA, Tavallai S, Hamed HA, Cruickshanks N, Dent P (2014) The role of cell signalling in the crosstalk between autophagy and apoptosis. Cell Signal 26:549–555
Liu X, Sun J (2010) Endothelial cells dysfunction induced by silica nanoparticles through oxidative stress via JNK/P53 and NF-κB pathways. Biomaterials 31:8198–8209
Mladenovic Z, Johansson A, Willman B, Shahabi K, Bjorn E, Ransjo M (2014) Soluble silica inhibits osteoclast formation and bone resorption in vitro. Acta Biomater 10:406–418
Li Z, Liu F, Zhang L, Cao Y, Shao Y, Wang X, Jiang X, Chen Z (2018) Neuroserpin restores autophagy and promotes functional recovery after acute spinal cord injury in rats. Mol Med Rep 17:2957–2963
Novosel EC, Kleinhans C, Kluger PJ (2011) Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev 63:300–311
Santos MI, Unger RE, Sousa RA, Reis RL, Kirkpatrick CJ (2009) Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone-starch scaffold and the in vitro development of vascularization. Biomaterials 30:4407–4415
Grellier M, Bordenave L, Amédée J (2009) Cell-to-cell communication between osteogenic and endothelial lineages: implications for tissue engineering. Trends Biotechnol 27:562–571
Pruksa S, Siripinyanond A, Powell JJ, Jugdaohsingh R (2014) Silicon balance in human volunteers; a pilot study to establish the variance in silicon excretion versus intake. Nutr Metab (Lond) 11(1):4
Li H, Ke X, Ni K, Kai L, Jiang C (2014) Silicate bioceramics enhanced vascularization and osteogenesis through stimulating interactions between endothelia cells and bone marrow stromal cells. Biomaterials 35:3803–3818
Zhai W, Lu H, Chen L, Lin X, Huang Y, Dai K, Naoki K, Chen G, Chang J (2012) Silicate bioceramics induce angiogenesis during bone regeneration. Acta Biomater 8:341–349
Duan J, Yu Y, Li Y, Yu Y, Sun Z (2013) Cardiovascular toxicity evaluation of silica nanoparticles in endothelial cells and zebrafish model. Biomaterials 34:5853–5862
Kim KH, Lee MS (2014) Autophagy--a key player in cellular and body metabolism. Nat Rev Endocrinol 10:322–337
Guo C, Yang M, Jing L, Wang J, Yu Y, Li Y, Duan J, Zhou X, Li Y, Sun Z (2016) Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling. Int J Nanomedicine 11:5257–5276
Wang J, Yu Y, Lu K, Yang M, Li Y, Zhou X, Sun Z (2017) Silica nanoparticles induce autophagy dysfunction via lysosomal impairment and inhibition of autophagosome degradation in hepatocytes. Int J Nanomedicine 12:809–825
Asweto CO, Wu J, Alzain MA, Hu H, Andrea S, Feng L, Yang X, Duan J, Sun Z (2017) Cellular pathways involved in silica nanoparticles induced apoptosis: a systematic review of in vitro studies. Environ Toxicol Pharmacol 56:191–197
Nakamura S, Yoshimori T (2017) New insights into autophagosome-lysosome fusion. J Cell Sci 130:1209–1216
Mukhopadhyay S, Panda PK, Sinha N, Das DN, Bhutia SK (2014) Autophagy and apoptosis: where do they meet? Apoptosis 19:555–566
Acknowledgments
We appreciate the instruction work by professor Changsheng Liu and his fellow from East China University of Science and Technology.
Funding
This work was supported by the Key Project of Natural Science Foundation of China (Grant No. 31330028)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Liu, S., Cao, Y. et al. High Concentration of Sodium Metasilicate Impairs Autophagic Flux and Induces Apoptosis in Human Umbilical Vein Endothelial Cells. Biol Trace Elem Res 191, 88–97 (2019). https://doi.org/10.1007/s12011-018-1608-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1608-3