Abstract
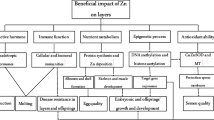
The role of in ovo zinc (Zn) injection in improving the embryonic development in eggs from Zn-deficient hens, via epigenetic and antioxidant mechanisms, was examined. A completely randomized design involving a 1 (the non-injected control) + 1 (the injected control with sterilized water) + 2 (Zn source) × 2 (Zn level) factorial arrangement of treatments was used. The two injected Zn sources were inorganic Zn sulfate and organic Zn-lysine chelate with a moderate chelation strength, and the two injected Zn levels were 50 and 100 μg Zn/egg. In ovo Zn injection decreased (P < 0.05) embryonic mortality, and increased (P < 0.05) hatchability and healthy chick ratio. In ovo Zn injection increased (P < 0.05) embryonic tibia Zn content, but had no effect (P > 0.05) on copper (Cu)- and Zn-containing superoxide dismutase (CuZnSOD) activities and metallothionein IV (MT4) levels or their mRNA expression levels and malondialdehyde (MDA) levels in the embryonic liver. In ovo Zn injection had no effect (P > 0.05) on the global level of DNA methylation or DNA methylation and histone 3 lysine 9 (H3K9) acetylation levels of the MT4 promoter in the embryonic liver. However, the organic Zn had higher (P < 0.05) levels of DNA methylation and H3K9 acetylation than inorganic Zn. These data demonstrate that in ovo Zn injection improved the embryonic development, and the organic Zn was more effective than inorganic Zn in enhancing DNA methylation and H3K9 acetylation in the liver MT4 promoter, but the precise mechanisms require further investigations.
Similar content being viewed by others
References
Zhu YW, Li WX, Lu L, Zhang LY, Ji C, Lin X, Liu HC, Odle J, Luo XG (2017) Impact of maternal heat stress in conjunction with dietary zinc supplementation on hatchability, embryonic development, and growth performance in offspring broilers. Poult Sci 96(7):2351–2359. https://doi.org/10.3382/ps/pew481
Yin LJ, Zhang Y, Lv PP, He WH, Wu YT, Liu AX, Ding GL, Dong MY, Qu F, Xu CM, Zhu XM, Huang HF (2012) Insufficient maintenance DNA methylation is associated with abnormal embryonic development. BMC Med 10(1):26. https://doi.org/10.1186/1741-7015-10-26
Vandegehuchte MB, Lemiere F, Janssen CR (2009) Quantitative DNA-methylation in Daphnia magna and effects of multigeneration Zn exposure. Comp Biochem Phys C 150(3):343–348. https://doi.org/10.1016/J.Cbpc.2009.05.014
Huang YL, Lu L, Li SF, Luo XG, Liu B (2009) Relative bioavailabilities of organic zinc sources with different chelation strengths for broilers fed a conventional corn-soybean meal diet. J Anim Sci 87(6):2038–2046. https://doi.org/10.2527/jas.2008-1212
Huang YL, Lu L, Luo XG, Liu B (2007) An optimal dietary zinc level of broiler chicks fed a corn-soybean meal diet. Poult Sci 86(12):2582–2589. https://doi.org/10.3382/ps.2007-00088
Huang YL, Lu L, Xie JJ, Li SF, Li XL, Liu SB, Zhang LY, Xi L, Luo XG (2013) Relative bioavailabilities of organic zinc sources with different chelation strengths for broilers fed diets with low or high phytate content. Anim Feed Sci Technol 179(1-4):144–148. https://doi.org/10.1016/j.anifeedsci.2012.10.010
Liu ZH, Lu L, Li SF, Zhang LY, Xi L, Zhang KY, Luo XG (2011) Effects of supplemental zinc source and level on growth performance, carcass traits, and meat quality of broilers. Poult Sci 90(8):1782–1790. https://doi.org/10.3382/ps.2010-01215
Liao XD, Li A, Lu L, Liu SB, Li SF, Zhang LY, Wang GY, Luo XG (2013) Optimal dietary zinc levels of broiler chicks fed a corn–soybean meal diet from 22 to 42 days of age. Anim Prod Sci 53(5):388–394. https://doi.org/10.1071/AN12291
Liu S, Li S, Lu L, Xie J, Zhang L, Wang R, Luo X (2013) The effectiveness of zinc proteinate for chicks fed a conventional corn-soybean meal diet. J Appl Poult Res 22(3):396–403. https://doi.org/10.3382/japr.2012-00564
Shen SF, Wang RL, Lu L, Li SF, Liu SB, Xie JJ, Zhang LY, Wang ML, Luo XG (2013) Effect of intravenously injected zinc on tissue zinc and metallothionein gene expression of broilers. Brit Poultry Sci 54:381–390
Li WX, Ma XY, Lu L, Zhang LY, Luo XG (2015) Relative bioavailability of tribasic zinc sulfate for broilers fed a conventional corn-soybean meal diet. J Integr Agr 14(10):2042–2049. https://doi.org/10.1016/S2095-3119(15)61033-4
Liu ZH, Lu L, Wang RL, Lei HL, Li SF, Zhang LY, Luo XG (2015) Effects of supplemental zinc source and level on antioxidant ability and fat metabolism-related enzymes of broilers. Poult Sci 94(11):2686–2694. https://doi.org/10.3382/ps/pev251
Suo HQ, Lu L, Zhang LY, Zhang XY, Li H, Lu YF, Luo XG (2015) Relative bioavailability of zinc-methionine chelate for broilers fed a conventional corn-soybean meal diet. Biol Trace Elem Res 165(2):206–213. https://doi.org/10.1007/s12011-015-0252-4
Majumder S, Ghoshal K, Li Z, Bo Y, Jacob ST (1999) Silencing of metallothionein-I gene in mouse lymphosarcoma cells by methylation. Oncogene 18(46):6287–6295. https://doi.org/10.1038/sj.onc.1203004
Kadam MK, Barekatain MR, Bhanja SK, Iji PA (2013) Prospects of in ovo feeding and nutrient supplementation for poultry: the science and commercial applications—a review. J Sci Food Agric 93(15):3654–3661. https://doi.org/10.1002/jsfa.6301
Yair R, Uni Z (2011) Content and uptake of minerals in the yolk of broiler embryos during incubation and effect of nutrient enrichment. Poult Sci 90(7):1523–1531. https://doi.org/10.3382/Ps.2010-01283
Sun XM, Liao XD, Lu L, Zhang LY, Ma QG, Lin X, Luo XG (2017) Effect of in ovo zinc injection on the embryonic development, tissue zinc contents, antioxidation, and related gene expressions of broiler breeder eggs. J Integr Agr 16:60345–60347
Macalintal LM (2012) In Ovo selenium (Se) injection of incubating chicken eggs: effects on embryo viability, tissue Se concentration, lipid peroxidation, immune response and post hatch development. Doctoral dissertation thesis, University of Kentucky
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(−Delta Delta C) method. Methods 25(4):402–408. https://doi.org/10.1006/Meth.2001.1262
Zhu Y, Liao X, Lu L, Li W, Zhang L, Ji C, Lin X, Liu HC, Odle J, Luo X (2017) Maternal dietary zinc supplementation enhances the epigenetic-activated antioxidant ability of chick embryos from maternal normal and high temperatures. Oncotarget 8(12):19814–19824. https://doi.org/10.18632/oncotarget.15057
Liu S, Lu L, Li S, Xie J, Zhang L, Wang R, Luo X (2012) Copper in organic proteinate or inorganic sulfate form is equally bioavailable for broiler chicks fed a conventional corn-soybean meal diet. Biol Trace Elem Res 147(1-3):142–148. https://doi.org/10.1007/s12011-012-9329-5
Goel A, Bhanja SK, Mehra M, Mandal A, Pande V (2016) In ovo trace element supplementation enhances expression of growth genes in embryo and immune genes in post-hatch broiler chickens. J Sci Food Agric 96(8):2737–2745. https://doi.org/10.1002/jsfa.7438
Jose N, Elangovan AV, Awachat VB, Shet D, Ghosh J, David CG (2017) Response of in ovo administration of zinc on egg hatchability and immune response of commercial broiler chicken. J Anim Physiol Anim Nutr (Berl) 1–5. doi:https://doi.org/10.1111/jpn.12777
Joshua PP, Valli C, Balakrishnan V (2016) Effect of in ovo supplementation of nano forms of zinc, copper, and selenium on post-hatch performance of broiler chicken. Vet World 9(3):287–294. https://doi.org/10.14202/vetworld.2016.287-294
Chen ZX, Riggs AD (2011) DNA methylation and demethylation in mammals. J Biol Chem 286(21):18347–18353. https://doi.org/10.1074/jbc.R110.205286
Gregory PD, Wagner K, Horz W (2001) Histone acetylation and chromatin remodeling. Exp Cell Res 265(2):195–202. https://doi.org/10.1006/excr.2001.5187
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73(1):79–118. https://doi.org/10.1152/physrev.1993.73.1.79
Tian X, Diaz FJ (2013) Acute dietary zinc deficiency before conception compromises oocyte epigenetic programming and disrupts embryonic development. Dev Biol 376(1):51–61. https://doi.org/10.1016/j.ydbio.2013.01.015
Verdone L, Caserta M, Di Mauro E (2005) Role of histone acetylation in the control of gene expression. Biochem Cell Biol 83(3):344–353. https://doi.org/10.1139/o05-041
Acknowledgements
The present study was supported by the Agricultural Science and Technology Innovation Program (ASTIP-IAS08; Beijing, P.R. China), the Key International Cooperation Program of the National Natural Science Foundation of China (project no. 31110103916; Beijing, P.R. China), and the China Agriculture Research System (project no. CARS-41; Beijing, P.R. China). In addition, we would highly appreciate Dr. David Masters in the University of Western Australia for assisting with the English editing of this manuscript.
Author information
Authors and Affiliations
Contributions
X. Luo was responsible for all issues related to this paper. X. S. and L. L. were responsible for the planning of the study, sample analyses, collections, and statistical analyses of all data, as well as the manuscript writing. X. L. and L. Z. were involved in the sample collections. X. L. and Q. M. were involved in the experimental design and statistical analyses. All authors contributed to the writing of the manuscript and agreed with the final content.
Corresponding authors
Ethics declarations
All experimental procedures were approved by the Animal Management Committee (in charge of animal welfare) of the Institute of Animal Science, Chinese Academy of Agricultural Sciences (IAS-CAAS, Beijing, China) and performed in accordance with the guidelines. Ethical approval on animal survival was given by the Animal Ethics Committee of IAS-CAAS.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOC 273 kb)
Rights and permissions
About this article
Cite this article
Sun, X., Lu, L., Liao, X. et al. Effect of In Ovo Zinc Injection on the Embryonic Development and Epigenetics-Related Indices of Zinc-Deprived Broiler Breeder Eggs. Biol Trace Elem Res 185, 456–464 (2018). https://doi.org/10.1007/s12011-018-1260-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1260-y