Abstract
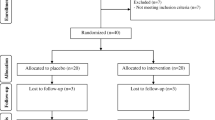
This study was conducted to evaluate the effects of selenium supplementation on gene expression related to insulin and lipid in infertile women with polycystic ovary syndrome (PCOS) candidate for in vitro fertilization (IVF). This randomized double-blind, placebo-controlled trial was conducted among 40 infertile women with PCOS candidate for IVF. Subjects were randomly allocated into two groups to intake either 200-μg selenium (n = 20) or placebo (n = 20) per day for 8 weeks. Gene expression levels related to insulin and lipid were quantified in lymphocytes of women with PCOS candidate for IVF with RT-PCR method. Results of RT-PCR demonstrated that after the 8-week intervention, compared with the placebo, selenium supplementation upregulated gene expression of peroxisome proliferator-activated receptor gamma (PPAR-γ) (1.06 ± 0.15-fold increase vs. 0.94 ± 0.18-fold reduction, P = 0.02) and glucose transporter 1 (GLUT-1) (1.07 ± 0.20-fold increase vs. 0.87 ± 0.18-fold reduction, P = 0.003) in lymphocytes of women with PCOS candidate for IVF. In addition, compared with the placebo, selenium supplementation downregulated gene expression of low-density lipoprotein receptor (LDLR) (0.88 ± 0.17-fold reduction vs. 1.05 ± 0.22-fold increase, P = 0.01) in lymphocytes of women with PCOS candidate for IVF. We did not observe any significant effect of selenium supplementation on gene expression levels of lipoprotein(a) [LP(a)] in lymphocytes of women with PCOS candidate for IVF. Overall, selenium supplementation for 8 weeks in lymphocytes of women with infertile PCOS candidate for IVF significantly increased gene expression levels of PPAR-γ and GLUT-1 and significantly decreased gene expression levels of LDLR, but did not affect LP(a).
Clinical trial registration number: http://www.irct.ir: IRCT201704245623N113.





Similar content being viewed by others
Change history
28 February 2020
The Editors-in-Chief are currently investigating this article [Zadeh Modarres, S., Heidar, Z., Foroozanfard, F. et al. The Effects of Selenium Supplementation on Gene Expression Related to Insulin and Lipid in Infertile Polycystic Ovary Syndrome Women Candidate for In Vitro Fertilization: a Randomized, Double-Blind, Placebo-Controlled Trial. Biol Trace Elem Res 183, 218–225 (2018). https://doi.org/10.1007/s12011-017-1148-2] as concerns have been raised about integrity of the clinical trial reported here. There is also an ongoing investigation by the Iranian National Committee for Ethics in Biomedical Researches. Further editorial action will be taken as appropriate once the investigation into the concerns is complete and all parties have been given an opportunity to respond in full.
References
Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO (2004) The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab 89:2745–2749
Fulghesu AM, Villa P, Pavone V et al (1997) The impact of insulin secretion on the ovarian response to exogenous gonadotropins in polycystic ovary syndrome. J Clin Endocrinol Metab 82:644–648
Dale PO, Tanbo T, Haug E, Abyholm T (1998) The impact of insulin resistance on the outcome of ovulation induction with low-dose follicle stimulating hormone in women with polycystic ovary syndrome. Hum Reprod 13:567–570
Uno K, Katagiri H, Yamada T et al (2006) Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science 312:1656–1659
Puttabyatappa M, Vandevoort CA, Chaffin CL (2010) hCG-induced down-regulation of PPARgamma and liver X receptors promotes periovulatory progesterone synthesis by macaque granulosa cells. Endocrinology 151:5865–5872
Carlioglu A, Kaygusuz I, Karakurt F et al (2014) The platelet activating factor acetyl hydrolase, oxidized low-density lipoprotein, paraoxonase 1 and arylesterase levels in treated and untreated patients with polycystic ovary syndrome. Arch Gynecol Obstet 290:929–935
Liu ML, Ylitalo K, Salonen R, Salonen JT, Taskinen MR (2004) Circulating oxidized low-density lipoprotein and its association with carotid intima-media thickness in asymptomatic members of familial combined hyperlipidemia families. Arterioscler Thromb Vasc Biol 24:1492–1497
Navarro-Alarcon M, Cabrera-Vique C (2008) Selenium in food and the human body: a review. Sci Total Environ 400:115–141
Jamilian M, Samimi M, Afshar Ebrahimi F et al (2017) Effects of selenium supplementation on gene expression levels of inflammatory cytokines and vascular endothelial growth factor in patients with gestational diabetes. Biol Trace Elem Res. https://doi.org/10.1007/s12011-017-1045-8
El Dib R, Gameiro OL, Ogata MS et al (2015) Zinc supplementation for the prevention of type 2 diabetes mellitus in adults with insulin resistance. Cochrane database Syst rev:CD005525
Asemi Z, Jamilian M, Mesdaghinia E, Esmaillzadeh A (2015) Effects of selenium supplementation on glucose homeostasis, inflammation, and oxidative stress in gestational diabetes: randomized, double-blind, placebo-controlled trial. Nutrition 31:1235–1242
Lu J, Holmgren A (2009) Selenoproteins. J Biol Chem 284:723–727
Donma MM, Donma O (2016) Promising link between selenium and peroxisome proliferator activated receptor gamma in the treatment protocols of obesity as well as depression. Med Hypotheses 89:79–83
Gao X, Zhang Z, Li Y et al (2016) Selenium deficiency deteriorate the inflammation of S. aureus infection via regulating NF-kappaB and PPAR-gamma in mammary gland of mice. Biol Trace Elem Res 172:140–147
Vunta H, Davis F, Palempalli UD et al (2007) The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12,14-prostaglandin J2 in macrophages. J Biol Chem 282:17964–17973
Hussein O, Rosenblat M, Refael G, Aviram M (1997) Dietary selenium increases cellular glutathione peroxidase activity and reduces the enhanced susceptibility to lipid peroxidation of plasma and low-density lipoprotein in kidney transplant recipients. Transplantation 63:679–685
Diamanti-Kandarakis E, Dunaif A (2012) Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 33:981–1030
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81:19–25
Hatch R, Rosenfield RL, Kim MH, Tredway D (1981) Hirsutism: implications, etiology, and management. Am J Obstet Gynecol 140:815–830
Gmelig-Meyling F, Waldmann TA (1980) Separation of human blood monocytes and lymphocytes on a continuous Percoll gradient. J Immunol Methods 33:1–9
Burk RF, Norsworthy BK, Hill KE, Motley AK, Byrne DW (2006) Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol Biomark Prev 15:804–810
Aldosary BM, Sutter ME, Schwartz M, Morgan BW (2012) Case series of selenium toxicity from a nutritional supplement. Clin Toxicol (Phila) 50:57–64
Asemi Z, Foroozanfard F, Hashemi T, Bahmani F, Jamilian M, Esmaillzadeh A (2015) Calcium plus vitamin D supplementation affects glucose metabolism and lipid concentrations in overweight and obese vitamin D deficient women with polycystic ovary syndrome. Clin Nutr 34:586–592
Foroozanfard F, Jamilian M, Bahmani F et al (2015) Calcium plus vitamin D supplementation influences biomarkers of inflammation and oxidative stress in overweight and vitamin D-deficient women with polycystic ovary syndrome: a randomized double-blind placebo-controlled clinical trial. Clin Endocrinol 83:888–894
Wang YX, Zhu WJ, Xie BG (2014) Expression of PPAR-gamma in adipose tissue of rats with polycystic ovary syndrome induced by DHEA. Mol Med Rep 9:889–893
Akyurek N, Aycan Z, Cetinkaya S, Akyurek O, Yilmaz Agladioglu S, Ertan U (2013) Peroxisome proliferator activated receptor (PPAR)-gamma concentrations in childhood obesity. Scand J Clin Lab Invest 73:355–360
Mueller AS, Pallauf J (2006) Compendium of the antidiabetic effects of supranutritional selenate doses. In vivo and in vitro investigations with type II diabetic db/db mice. J Nutr Biochem 17:548–560
TJ X, Liu Y, Yuan B (2011) Effect of insulin in combination with selenium on Irs/PI3K-mediated GLUT4 expression in cardiac muscle of diabetic rats. Eur Rev Med Pharmacol Sci 15:1452–1460
Nido SA, Shituleni SA, Mengistu BM et al (2016) Effects of selenium-enriched probiotics on lipid metabolism, antioxidative status, histopathological lesions, and related gene expression in mice fed a high-fat diet. Biol Trace Elem Res 171:399–409
Zhou F, Shi LB, Zhang SY (2017) Ovarian fibrosis: a phenomenon of concern. Chin Med J 130:365–371
Strakova N, Ehrmann J, Bartos J, Malikova J, Dolezel J, Kolar Z (2005) Peroxisome proliferator-activated receptors (PPAR) agonists affect cell viability, apoptosis and expression of cell cycle related proteins in cell lines of glial brain tumors. Neoplasma 52:126–136
Legro RS (2003) Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev 24:302–312
Cassar S, Teede HJ, Harrison CL, Joham AE, Moran LJ, Stepto NK (2015) Biomarkers and insulin sensitivity in women with PCOS: characteristics and predictive capacity. Clin Endocrinol 83:50–58
Unfer V, Porcaro G (2014) Updates on the myo-inositol plus D-chiro-inositol combined therapy in polycystic ovary syndrome. Expert Rev Clin Pharmacol 7:623–631
Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E (2006) Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr 84:762–773
Brown BG, Zhao XQ, Chait A et al (2001) Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 345:1583–1592
Hercberg S (2006) The SU.VI.MAX study, a randomized, placebo-controlled trial on the effects of antioxidant vitamins and minerals on health. Ann Pharm Fr 64:397–401
Li R, Xu X, Chen C et al (2015) CYP2J2 Attenuates metabolic dysfunction in diabetic mice by reducing hepatic inflammation via the PPAR gamma. Am J Physiol Endocrinol Metab 308:E270–E282
Zhang W, Zhang R, Wang T et al (2014) Selenium inhibits LPS-induced pro-inflammatory gene expression by modulating MAPK and NF-kappaB signaling pathways in mouse mammary epithelial cells in primary culture. Inflammation 37:478–485
Gandhi UH, Kaushal N, Ravindra KC et al (2011) Selenoprotein-dependent up-regulation of hematopoietic prostaglandin D2 synthase in macrophages is mediated through the activation of peroxisome proliferator-activated receptor (PPAR) gamma. J Biol Chem 286:27471–27482
Ghaffari T, Nouri M, Irannejad E, Rashidi MR (2011) Effect of vitamin e and selenium supplement on paraoxonase-1 activity, oxidized low density lipoprotein and antioxidant defense in diabetic rats. Bioimpacts 1:121–128
Leborgne L, Pakala R, Dilcher C et al (2005) Effect of antioxidants on atherosclerotic plaque formation in balloon-denuded and irradiated hypercholesterolemic rabbits. J Cardiovasc Pharmacol 46:540–547
Alissa EM, Ahmed WH, Al-ama N, Ferns GA (2008) Selenium status and cardiovascular risk profile in healthy adult Saudi males. Molecules 14:141–159
Inoue T, Uchida T, Kamishirado H, Takayanagi K, Hayashi T, Morooka S (2001) Clinical significance of antibody against oxidized low density lipoprotein in patients with atherosclerotic coronary artery disease. J Am Coll Cardiol 37:775–779
Galle J, Hansen-Hagge T, Wanner C, Seibold S (2006) Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis 185:219–226
Kuzuya M, Nakamura K, Sasaki T, Cheng XW, Itohara S, Iguchi A (2006) Effect of MMP-2 deficiency on atherosclerotic lesion formation in apoE-deficient mice. Arterioscler Thromb Vasc Biol 26:1120–1125
Auge N, Maupas-Schwalm F, Elbaz M et al (2004) Role for matrix metalloproteinase-2 in oxidized low-density lipoprotein-induced activation of the sphingomyelin/ceramide pathway and smooth muscle cell proliferation. Circulation 110:571–578
Ghaffari T, Nouri M, Saei AA, Rashidi MR (2012) Aldehyde and xanthine oxidase activities in tissues of streptozotocin-induced diabetic rats: effects of vitamin E and selenium supplementation. Biol Trace Elem Res 147:217–225
Ozturk IC, Batcioglu K, Karatas F, Hazneci E, Genc M (2008) Comparison of plasma malondialdehyde, glutathione, glutathione peroxidase, hydroxyproline and selenium levels in patients with vitiligo and healthy controls. Indian J Dermatol 53:106–110
Rohr-Udilova N, Sieghart W, Eferl R et al (2012) Antagonistic effects of selenium and lipid peroxides on growth control in early hepatocellular carcinoma. Hepatology 55:1112–1121
Funding
This study was founded by a grant from the vice-chancellor for research, Shahid Beheshti, University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
ZA contributed in conception, design, statistical analysis, and drafting of the manuscript. SZ, ZH, FF, ZR, and EA contributed in data collection and manuscript drafting. All authors approved the final version for submission. ZA supervised the study.
Corresponding author
Ethics declarations
This study was conducted in accordance with the Declaration of Helsinki, and informed consent was obtained from all participants. The research was approved by the ethics committee of Shahid Beheshti University of Medical Sciences.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zadeh Modarres, S., Heidar, Z., Foroozanfard, F. et al. The Effects of Selenium Supplementation on Gene Expression Related to Insulin and Lipid in Infertile Polycystic Ovary Syndrome Women Candidate for In Vitro Fertilization: a Randomized, Double-Blind, Placebo-Controlled Trial. Biol Trace Elem Res 183, 218–225 (2018). https://doi.org/10.1007/s12011-017-1148-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-017-1148-2