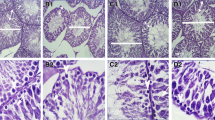
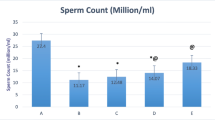
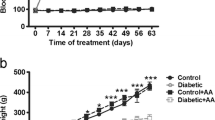
Abstract
Fluoride toxicity is known to pose infertility in fluoride-intoxicated animals as well as in people residing in fluoride endemic zones. The present study addresses the degree of impairments caused due to co-exposure of high fluoride toxicity in diabetic mice. Swiss mice, Mus musculus, were subjected to fluoride toxicity by providing fluoride-supplemented drinking water (600 ppm NaF) for a period of 30 days after the confirmation of streptozotocin-induced diabetes(STZ, 50 mg/kgbw). Consequently, aggravated hyperglycemia and tissue fluoride accumulation were witnessed in fluoride-intoxicated diabetic mice; later, these toxicated mice were treated with ginseng extract (GE) and banaba leaf extract, (BLE) at dose of 150 mg/kgbw/day alone and in combination for 15 and 30-day duration to check the efficacy of phytoextracts in reversing the toxicity. The spermatological indices studied, such as sperm density, motility, viability and morphology as well as the testicular biochemical parameters showed enhanced impairment in reproductive status of fluoride-intoxicated diabetic mice. Further, 15-days administration of GE and BLE in combination at a dose of 150 mg/kgbw/day was found to be beneficial in normalizing the alterations observed upon fluoride intoxication to diabetic mice. However, the correlates showed moderate association between blood glucose levels and the spermatological as well as biochemical indices wherein the tissue fluoride levels correlate least.




Similar content being viewed by others
References
Pushpalatha T, Srinivas M, Reddy SP (2005) Exposure to high fluoride concentration in drinking water will affect spermatogenesis and steroidogenesis in male albino rats. Biometals 18:207–212
Kumar A, Susheela AK (1994) Ultrastructural studies of spermiogenesis in rabbit exposed to chronic fluoride toxicity. Int J Fertil Menopausal Stud 39:164–171
Chinoy NJ, Sharma A (1998) Amelioration of fluoride toxicity by vitamins E and D in reproductive functions of male mice. Fluoride 31:203–216
Ghosh D, Das SS, Maiti R, Jana D, Das U (2002) Testicular toxicity in sodium fluoride treated rats: association with oxidative stress. Reprod Toxicol 16:385–390
Kumar A, Susheela AK (1995) Effects of chronic fluoride toxicity on the morphology of ductus epididymis and the maturation of spermatozoa of rabbit. Int J Exp Pathol 76:1–11
Narayana M, Chinoy N (1994) Effect of fluoride on rat testicular steroidogenesis. Fluoride 27:7–12
Izquierdo-Vega JA, Sánchez-Gutiérrez M, Del Razo LM (2008) Decreased in vitro fertility in male rats exposed to fluoride-induced oxidative stress damage and mitochondrial transmembrane potential loss. Toxicol Appl Pharmacol 230:352–357
Sprando RL, Collins TF, Black T, Olejnik N, Rorie J (1998) Testing the potential of sodium fluoride to affect spermatogenesis: a morphometric study. Food Chem Toxicol 36:1117–1124
Basha PM, Saumya SM (2013a) Influence of fluoride on streptozotocin induced diabetic nephrotoxicity in mice: protective role of Asian ginseng (Panax ginseng) & banaba (Lagerstroemia speciosa) on mitochondrial oxidative stress. Indian J Med Res 137:370–379
Basha PM, Saumya SM (2013b) Suppression of mitochondrial oxidative phosphorylation and TCA enzymes in discrete brain regions of mice exposed to high fluoride: amelioration by Panax ginseng (ginseng) and Lagerstroemia speciosa (banaba) extracts. Cell Mol Neurobiol 33:453–464
Saumya SM, Basha PM (2011) Antigenotoxic effect of ginseng and banaba on fluoride toxicated streptozotocin induced mice. J Cytol Genet 12:51–60
Hanhijarvi H, Penttila I, Pekkarinen A, Hakulinen A (1974) The effect of age on free ionized plasma fluoride concentrations in patients from artificially fluoridated and non-fluoridated drinking water communities. Proc Finn Dent Soc 3:25–34
Dote T, Kono K, Usuda K, Nishiura H, Tagawa T, Miyata K, Shimahara M, Hashiguchi N, Senda J, Tanaka Y (2000) Toxicokinetics of intravenous fluoride in rats with renal damage caused by high-dose fluoride exposure. Int Arch Occupy Environ Hlth 73(9):S90–S92
Mosher WD, Pratt WF (1991) Fecundity and infertility in the United States: incidence and trends. Fertil Steril 56:192–193
Mallick C, Mandal S, Barik B, Bhattacharya A, Ghosh D (2007) Protection of testicular dysfunctions by MTEC, a formulated herbal drug, in streptozotocin induced diabetic rat. Biol and Pharm Bull 30:84–90
Rabbani SI, Devi K, Khanam S (2010) Role of pioglitazone with metformin or glimepiride on oxidative stress-induced nuclear damage and reproductive toxicity in diabetic rats. Malays J Med Sci 7:3–11
Trivedi M, Verma R, Chinoy N (2006) Amelioration by black tea of changes induced by sodium fluoride in protein content of liver and kidney in mice. Fluoride 39:269–273
Ojo O, Ladeji O, Nadro M (2007) Studies of the antioxidative effects of green and black tea extracts in rats. J Med Food 10:345–349
Nocerino E, Amato M, Izzo AA (2000) The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia 71:1–5
Kakuda T, Sakane I, Takihara T, Ozaki Y, Takeuchi H, Kuroyanagi M (1996) Hypoglycemic effect of extracts from Lagerstroemia speciosa L. leaves in genetically diabetic KK-AY mice. Biosci Biotechnol Biochem 60:204–208
Saumya SM, Basha PM (2011) In vitro evaluation of free radical scavenging activities of Panax ginseng and Lagerstroemia speciosa: a comparative analysis. Int J Pharm Pharm Sci 3:165–169
Nabavi SM, Nabavi SF, Loizzo MR, Sureda A, Amani MA, Moghaddam AH (2012) Cytoprotective effect of silymarin against sodium fluoride-induced oxidative stress in rat erythrocytes. Fluoride 45:27–34
Inkielewicz I, Krechniak J (2003) Fluoride content in soft tissues and urine of rats exposed to sodium fluoride in drinking water. Fluoride 36:263–266
Akbarsha MA, Kadalmani B, Girija R, Faridha A, Hamid KS (2001) Spermatotoxic effect of carbendazim. Indian J Exp Biol 39:921–924
Prasad MR, Chinoy NJ, Kadam KM (1972) Changes in succinic dehydrogenase levels in the rat epididymis under normal and altered physiologic conditions. Fertil and Steril 23:186–190
World Health Organization (1999) Laboratory manual for examination of human semen and semen-cervical mucus interaction. 4th Edn. The Press Syndicate of the University of Cambridge, Cambridge
Nahas S, Hondt HA, Abdou HA (1989) Chromosome aberrations in spermatogonia and sperm abnormalities in Curacron-treated mice. Mutat Res 222:409–414
Mori K, Kaido M, Fujishiro K, Inoue N, Koide O, Hori H, Tanaka I (1991) Dose dependent effects of inhaled ethylene oxide on spermatogenesis in rats. Br J Ind Med 48:270–274
Okamura A, Kamijima M, Shibata E, Ohtani K, Takagi K, Ueyama J, Watanabe Y, Omura M, Wang H, Ichihara G, Kondo T, Nakajima T (2005) A comprehensive evaluation of the testicular toxicity of Dichlorvos in Wistar rats. Toxicol 213:129–137
Larson JL, Miller DJ (1999) Simple histochemical stain for acrosome on sperm from several species. Mol Reprod Dev 52:445–449
Zak B (1957) Simple rapid microtechnic for serum total cholesterol. Am J Clin Pathol 27:583–588
Seifter S, Dayton S, Novic B, Muntwyler E (1950) The estimation of glycogen with the anthrone reagent. Arch Biochem 25:191–200
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Mann T (1946) Studies on the metabolism of semen, fructose as a normal constituent of seminal plasma site of formation and function of fructose in semen. Biochem J 40:481–491
Chinoy NJ, Mehta D, Jhala DD (2006) Effects of fluoride ingestion with protein deficient or protein enriched diets on sperm function of mice. Fluoride 39:11–16
Chinoy NJ (1995) Role of fluoride in animal systems: a review. In: Prakash R, Sood P (eds) Toxicity and monitoring of xenobiotics. Venus Publishing House, New Delhi, pp. 13–30
Liu H, Niu R, Wang J, He Y, Wang J (2008) Changes caused by fluoride and lead in energy metabolic enzyme activities in the reproductive system of male offspring rats. Fluoride 4:184–191
Zakrezewska H, Udala J, Blaszczykb B (2002) In vitro influence of sodium fluoride on ram semen quality and enzyme activities. Fluoride 35:153–160
Huang C, Niu RY, Wang JD (2007) Toxic effects of sodium fluoride on reproductive function in male mice. Fluoride 40:162–168
Perreault SD, Cancel AM (2001) Significance of incorporating measures of sperm production and function into rat toxicology studies. Reproduction 121:207–216
Dvoráková-Hortová K, Sandera M, Jursová M, Vasinová J, Peknicová J (2008) The influence of fluorides on mouse sperm capacitation. Anim Reprod Sci 108:157–170
Verma PK, Sharma A, Mathur A, Sharma PS, Gupta RS, Joshi SC, Dixit VP (2002) Effect of Sarcostemma acidum stem extract on spermatogenesis in male albino rats. Asian J Androl 4:43–47
Bedwal RS, Edwards MS, Katoch M, Bahugana A, Dewan R (1994) Histological and biochemical changes in testis of zinc deficient BALB/c strain of mice. Indian J Exp Biol 32:243–247
Dixit VP, Joshi S (1982) Effect of chronic administration of garlic (Allium sativum) on testicular function. Indian J Exp Biol 20:534–536
Cui LX, Jiang CX, Wang XL, Chen XM (2003) Experimental study on effect of fluoride on reproductive system of male rats. Chin J Epidemiol 22:195–197 [in Chinese]
Dare BJ, Oyeniyi F, Olaniyan OT (2014) Role of antioxidant in testicular integrity. Annu Res Rev Biol 4(7):998–1023
Chu GX, Chen X (1990) Anti-lipid peroxidation and protection of ginsenosides against cerebral ischemia-reperfusion injuries in rats. Zhongguo Yao Li Xue Bao 11:119–123
Hong B, Ji YH, Hong JH, Nam KY, Ahn TY (2002) A double-blind crossover study evaluating the efficacy of Korean red ginseng in patients with erectile dysfunction: a preliminary report. J Urol 168:2070–2073
Salvati G, Genovesi G, Marcellini L, Paolini P, De Nuccio I, Pepe M, Re M (1996) Effects of Panax ginseng C.A. Meyer saponins on male fertility. Panminerva Med 38:249–254
Van Kampen J, Robertson H, Hagg T, Drobitch R (2003) Neuroprotective actions of the ginseng extract G115 in two rodent models of Parkinson’s disease. Exp Neurol 184:21–29
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
SM, S., Mahaboob Basha, P. Fluoride Exposure Aggravates the Testicular Damage and Sperm Quality in Diabetic Mice: Protective Role of Ginseng and Banaba. Biol Trace Elem Res 177, 331–344 (2017). https://doi.org/10.1007/s12011-016-0893-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0893-y