Abstract
Fungi are a small but important part of the human microbiota and several fungi are familiar to the immune system, yet certain can cause infections in immunocompromised hosts and referred as opportunistic pathogens. The fungal coinfections in COVID-19 hosts with predisposing conditions and immunosuppressive medications are posing higher severity and death. The immunological counteraction (innate/adaptive immunity) is triggered when the PRRs on the host cells recognize the fungal PAMPs. However, in simultaneous infections (COVID-19 and fungal coinfection), the synergism of TLR and NLR may hyperactivate the immune cells which dramatically increase the cytokine level and generate cytokine storm. Fungal colonization in the human gut assists the development of microbiome assembly, ecology, and shaping immune response. However, SARS-CoV-2 infection represented unstable mycobiomes and long-term dysbiosis in a large proportion in COVID-19 patients. Normally, amphotericin B is considered as first-line treatment for invasive fungal infection. So, amphotericin B therapy is recommended in COVID-19 hosts with serious fungal infections. Still, the long-term corticosteroid supplementation prescribed in case of severe pneumonia and lower oxygen levels may result in systemic fungal infection in COVID-19 patients, eventually limiting the lifesaving benefits of available medications. Also, due to the evolution of fungal resistance to available antibiotics, the current treatments are becoming ineffective. Therefore, this review summarizes the concerns, needed to deal with the impending crises.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
From an estimated 1.5–5 million fungal species, nearly 500 species are considered as infectious to the human being. Despite that, every year, fungal infections give rise to 11.5 million severe cases and 1.5 million deaths, worldwide [6, 24]. Most of the fungi are the natural kin of the human microbiome but certain are morbific in immunocompromised hosts thus called opportunistic pathogens. The opportunistic pathogens afflict localized, moderate, and superficial infections in healthy humans. However, these notorious infections cause systemic morbidity and increased mortality in immune-suppressed conditions such as diabetes, lung disease, organ transplant, cancer, chemotherapy, and HIV infection [16, 24]. Besides, the use of broad-spectrum antibiotics, immunosuppressive drugs, and corticosteroid therapies are attributed to a substantial increase in people with weakened immunity, resulting in a higher risk of opportunistic fungal infections (Fig. 1). Even the recent COVID-19 pandemic observed the opportunistic fungal infection in COVID-positive hosts. Herewith, more severity and mortality associated with these infections are being witnessed in COVID-19-positive patients with predisposing factors such as ARDS, diabetes, mechanical ventilation, organ transplant recipients, antibiotics, and immunosuppressive therapies [27, 37]. Therefore, opportunistic fungal infection’s incidence has gained the attention of researchers around the world.
The genera of Aspergillus, Cryptococcus, Candida, Mucor, Saccharomyces, and Pneumocystis are responsible for more than 75% mortality among all invasive fungal deaths worldwide [6]. Herein, the fungal coinfection in COVID-19 patients poses a serious challenge to the global health care system (Table 1). Cases of aspergillosis in COVID-19 patients were reported in several retrospective studies from China during first half of the year 2020 [39, 42]. Beyond 14,000 cases of mucormycosis (so-called black fungus) were observed in India alone during the second wave of COVID-19 [27]. Moreover, the morbidity and mortality associated with aspergillosis, candidiasis, and Pneumocystis pneumonia in COVID-19 patients appraised more severity in European countries, viz., UK, France, Netherlands, Spain, and Germany [17, 18]. These testimonies of emerging fungal coinfection in COVID-19 hosts demand more vigilance of the scientific community to tackle imminent quandary. The present mini-review scrutinizes and highlights the knowledge gap in the incidence of opportunistic fungal infection, immune interaction, and the role of mycobiome on COVID-19 pathogenicity and gives a perspective to better describe and deal the epidemiological diseases.
Interaction of Immune System and Fungal Components
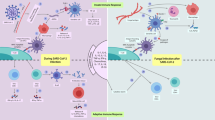
The cell wall components such as polysaccharides and the lipid molecules of fungal pathogens are known to stimulate the immune response. Also, the genetic makeup of the host participates vitally in shaping the immune defense against fungal encounters (Fig. 2). The PRRs present on the host cells (dendritic cells, macrophages, epithelial cells, fibroblasts, B and T cells) recognize the fungal PAMPs and initiate the immune response [31]. For example, the interactions of TLR-2 and mannan, TLR-4 and lipomannan, and TLR-9 and DNA (CpG) are known to trigger the immune response by stimulating MyD88 and type-I interferon production, respectively [10, 19]. The fungal β-glucans and α-mannans binding to CLRs dectin-1 and 2, respectively induce the NLR3 and NF-ĸB activation which further assist in Th1 and Th17 differentiation by stimulating cytokine production. Similarly, the DC-SIGN and MRs interaction with mannose molecules are reported to prompt IL-6, IL-10, and IL-17 production [24].
The interactions of fungal molecules and host receptors stimulate the innate and adaptive immune response. In that context, IFNγ (Th1) or IL-17 (Th17) from CD4 + T-cells are known for providing safeguard amidst fungal infection but the adaptive immunity to fungi is still unintelligible. The TLR-2, TLR-4, TLR-9, and NLRP-3 are activated by respiratory fungal infections, while SARS-CoV-2 and similar coronavirus only activate RIG-1, TLR-3, TLR-7, and NLRP-3. However, in simultaneous infections, the synergism of TLR and NLR can dramatically increase cytokine production. For instance, in critical COVID-19 cases, the TLR-4 (activated by fungal PAMPs) synergize with TLR-7 (activated by viral antigens) and TLR-2 (fungal activated) synergize with NLRP-3 (viral activated). As a result, COVID-19 and fungal coinfection stimulate synergetic innate action, leading to hyperinflammation and CSS (Fig. 3) [32].
The “cytokine storm” in severe COVID-19 cases is associated with the unique feature of SARS-CoV-2, where it stimulates NF-ĸB and blocks INF-1, which allow uninhibited replication of SARS-CoV-2. Further, in the fluids, abundance of immune cells and cytokines (IFN-α2, IFN-γ, IL-1, 2, 4, 7, 10, 12, and 17) are generated, where neutrophils infiltration is one of the notable pathologies of COVID-19 patients. Due to proinflammatory nature, these immune infiltrates start acting on their own inflammatory environment, resulting in hypercytokinemia (cytokine storm). This hyperinflammation generally inhibits viral propagation and causes excessive cell death, which might pose respiratory distress and eventually multiorgan failure [7, 34]. On the other hand, a signaling adaptor protein CARD9 is crucial for triggering an antifungal immune response. Thus, the CARD9-deficient humans or animals are more prone to fungal infections [9]. The study of human CARD9 deficiency together with invasive fungal disease protection might yield a unique insight into this comprehensive clinical dilemma.
Role of Fungal Biofilm and Genetics in Viral Coinfection
Biofilms can be formed by a variety of fungi, where hyphal cells may play a role in biofilm development. In fungal biofilms, filamentous hyphae and pseudohyphae may work in a similar way to promote metabolite exchange across biofilm cells. Biofilm formation on implanted devices is a primary cause of recurrent infection, apart from the fungal infection biology (8). An effective way to explain biofilm gene function is by identifying transcription factors which are needed for biofilm development. It is hypothesized that various gene products, including large family proteins (ALS and CFEM) and proteins similar to Hyr-1, may have combined actions in biofilm development [22]. For instance, biofilm cells of C. albicans over-express the genes for sulfur adsorption and methionine and cystine biosynthesis. It is also proposed that cystine-rich environment is required for the detoxification of ROS in biofilm cells [8, 26]. Also, various hyphal regulators and HSP-90 are known to control the biofilm cell release and create an extracellular matrix that is tolerant to a wide range of antimicrobials. Therefore, HSP-90 inhibitors might be an important player in lowering the rates of disseminated infection [30]. However, studies discover the role of C. parapsilosis’ (C. albicans’ orthologs) biofilm regulators and found the essential role of Bcr-1 and Efg-1 in biofilm development [8]. Overview of factors affecting fungal biofilm formation, resistance mechanism, important cellular elements/pathways, and potential inhibitors is depicted in Fig. 4.
Overview of factors influencing fungal biofilm development, biofilm resistance mechanism, important virulence factors, and cellular components/pathways involved in biofilm formation and tolerance. Potential inhibitors and their target are indicated by red inhibitory arrows (image created by Biorender app)
Biofilm possibly reduces viral sensitivity and facilitates virion survival by protecting against physicochemical stress through some mechanisms, i.e., immune regulation, desiccation in extracellular matrix, chemical resistance, and maintaining viral particles infectivity over time [38]. So, the fact that these viruses encased in biofilm maintained their vitality and infectivity suggests that the biofilm lifestyle does not hinder the viral spread and may even enhance it [14]. Also, biofilms may have a role in viral recombination, particularly in environments where different and infectious viruses (collective infectious unit) share a space together in the presence of extracellular proteins (enzymes) and genetic fragments [38]. Later, these viruses possibly break out of the biofilm and infect the same host cell, resulting in gene exchange and recombination. As a result of the aided cell coinfection by many viruses, new viral particles can evolve and develop, potentially spreading to other species [38]. Taken together, fungal pathogens that cause aspergillosis, candidiasis, cryptococcosis, mucormycosis, and other fungal infections have also evolved with unique adaptations to survive in hypoxic host conditions and greatly promote the chance for COVID-19 and fungal coinfection [1].
Fungal Detection and Therapeutic Strategies in COVID-19 Hosts
In general, microscopic inspection, culture, serological testing, AFST, and molecular assays are accessible methods to diagnose fungal infections. For instance, CT scans are being used to detect fungal infections in COVID-19 patients, primarily to visualize bone destruction, opacity, and periosteal thickening of the sinuses [43]. However, traditional culture and microscopic techniques are diagnostic keystones but deficit in sensitivity concerns. In molecular assays, the ITS sequencing is the first-line procedure for fungal species identification which may be utilized for the diagnosis of Mucorales. Besides, qPCR provides an assessment of fungal burden. Yet the molecular assays remain shortfall in diagnosis during mutation crisis and rare accessibility of modern serological testing methods in LDCs are major concerns. As if, the testing is an arcane profession that necessitates skilled specialists to detect fungal infections early, which is crucial to start the treatment on time.
The available treatments such as azoles (voriconazole, isavuconazole, and posaconazole), fluoropyrimidines, and echinocandins (micafungin, caspofungin, and anidulafungin) are used as inhibitors for fungal ergosterol synthesis, nucleic acid synthesis, and cell wall synthesis, respectively [17, 24]. Other than this, amphotericin B is considered as the first-line treatment for invasive fungal infection. So, the amphotericin B therapy is recommended in COVID-19 hosts with serious fungal infections. However, liposomal amphotericin B is preferred over amphotericin B, as if it is comparatively cheaper and less toxic [43]. Additionally, in severe mucormycosis cases, the adequate dose of intravenous isavuconazole and posaconazole along with liposomal amphotericin B is suggested by the Mycoses Study Group Education and Research Consortium and European Confederation of Medical Mycology [5]. Besides, sulfamethoxazole and trimethoprim combinations are advised to treat Pneumocystis infection in COVID-19 hosts. The corticosteroid supplementation may also be prescribed in case of severe pneumonia and lower oxygen levels.
However, long-term use of high doses of corticosteroids may result in systemic fungal infection in COVID-19 patients, eventually limiting the lifesaving benefits of these medications. Nevertheless, the emergence of resistance to these therapies is posing another global threat. For instance, the treatment of antifungals (voriconazole, caspofungin, and isavuconazole) did not affect the survivability of nearly 21% of COVID-19 patients [29]. Similarly, C. auris was isolated from 66% of candidemia cases in India, with a 60% mortality rate. Where fluconazole resistance was observed in all C. auris isolates, while 40% of them were found amphotericin B resistant [33]. In addition, the antifungal drugs and anti-inflammatory medications used in the treatment of COVID-19 patients with co-fungal infections are summarized in Table 2. The anti-inflammatory medicines perhaps prevent the deadly cytokine storm induced by SARS-CoV-2 infection.
Primarily recommended non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen are known to decrease IL-6 in sputum and human tissues [12]. On the other hand, the NSAIDs, mainly ibuprofen, may aggravate COVID-19 complications through the upregulation of the ACE-2 receptors in arteries, heart, intestine, kidney, and lungs, which might be utilized by SARS-CoV-2 as an entrance site into cells. Furthermore, by concealing fever and inflammation, NSAIDs may hinder the early diagnosis of COVID-19 [15]. However, in context of treating these fungal infections, the hyperbaric oxygen (HBO) therapy has been an adjuvant treatment as higher oxygen pressure may enhance neutrophil function and amphotericin B action, limiting spore germination and preventing fungal growth. Additionally, immunomodulation therapy may prove to be a scientific revolution to combat fungal infectivity, but many such targeted agents (mAbs, vaccines) are yet under clinical trials. Also, to prevent the adverse outcomes in COVID-19 patients, early diagnosis and screening for anti-fungal drug-resistant coinfection are necessary.
Effect on/of Mycobiomes in COVID-19 Infection
Fungi also share a significant proportion in the human gut microbiota, known as gut mycobiome. The gut fungi are known to be involved in the development of microbiome assembly, ecology, and shaping the immune response [35]. However, the COVID-19 patients represented the unstable mycobiomes and long-term dysbiosis in a large proportion (~ 30%) which might be due to SARS-CoV-2 infection [45]. The COVID-19 patients are also seen with an altered gut mycobiome enriched with C. albicans and diversified mycobiome configurations. Through the disease course, the bloom of opportunistic fungi such as A. flavus, C. auris, and C. albicans was detected in the fecal sample of COVID-19 hosts. Even after disease resolution, respiratory symptoms and pneumonia associated A. niger and A. flavus were observed in the feces of COVID-19 patients [44]. Infections of Aspergillus are noticed in the tracheal aspirates and respiratory tract secretions of COVID-19 patients, and more frequent coughing is observed as compared to non-infected hosts [42]. Therefore, a dysbiosis in the gut mycobiome might have a potential role in the dysregulated immunity of COVID-19 patients. It is known that ACE-2 receptors expressed on the cell surface (intestinal epithelium, esophagus, liver, and lungs) bind with SARS-CoV-2 and stimulate the infection. However, the microbiota generate metabolites during the fermentation process, which might block the ACE-2 or viral proteins and inhibit the viral spread [34]. In healthy people, Candida spp., Saccharomycetales, Cladosporium spp., and Aureobasidium spp. are the most common fungal population. But, as per the previous report, the mycobiome of COVID-19 hosts differs substantially from that of healthy individuals [28, 34].
Role of Probiotics and Gut Microbiota in Combating COVID-19
Probiotics have been shown to have anti-inflammatory, antiviral, and anti-allergic properties, as well as to reduce the time and rate of respiratory viral infections. Human microbiota can alter antiviral immunity, but the microbiota diversity varies with age [23]. As if, microbial population (microbiota) in neonates is in smaller number than adults and higher in young people than senior citizens. Therefore, senior citizens may be more prone to COVID-19 infection. Potential immune regulatory mechanism by probiotics comprises the stimulation of TLRs, alteration in gene expression, and biochemical cascade in host cells [21]. For instance, some biochemical signals could be communicated through the lower GI tract to another mucosal surface (respiratory tract), which might improve infection resistance. Also, the metabolites (short-chain fatty acids) produced by the gut microbiota regulate the mucosal site (intestine), which possibly help in restoring excretion of effecter cells, antibodies, and anti-inflammatory TNF-α and NF-ĸB in healthy hosts [34]. However, the lungs contain their microbiota, which interact with gut microbiota and indirectly affect the respiratory tracts’ immune response and inhibit the viral spread. Therefore, acute respiratory infections are perhaps accredited to dysbiosis in gut-lung microbiomes and intestinal dysbiosis to inflammation and weakened immune response to pathogens [11, 41]. The microbiota and COVID-19 infections have a straight connection and usage of probiotics may open new windows in managing the fungal disease. Yet, a lot remain to be learned regarding their potential clinical efficacy in COVID-19 incidents.
Conclusion and Future Perspective
More studies are needed to evaluate the links between environmental factors associated with fungal infections, available treatments, and co-fatalities. It is crucial to retain and rebuild nasal microflora and natural gut microbiota by lifestyle/dietary adjustments, prebiotics, probiotics, minerals, and vitamin supplementation. Furthermore, it is also essential to understand how the gut microbiota affects the immune system response against SARS-CoV-2. Commercial antifungals, antibiotics, and synthetic drugs are highly cytotoxic when used in heavy doses, thus limiting their utility. Therefore, the healthcare professionals must watch for patient-specific treatment procedures, medicine dosage, and related comorbidities. In that matter, novel antimicrobial agents from natural sources are one of the most promising therapeutic options for reducing antimicrobial resistance, as they pose minimal adverse effects. Besides, novel drug delivery systems such as nano-carriers, nanocarbon tubes, nanomesoporus, liposomes, as well as natural bioactive materials could be an optimal therapeutic approach. Along with that, several plant extracts, essence, and essential oils have shown excellent antibacterial, antifungal, and antiviral properties, which also can be utilized to deal with these clinical challenges. However, enough awareness of fungal infections and general personal precautions should be spread in public. The government interventions, policy implementation, and allocation of sufficient resources are also vital to combat this endemic.
Data Availability
The data generated and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- COVID-19:
-
Coronavirus disease-19
- HIV:
-
Human immunodeficiency virus
- ARDS:
-
Acute respiratory distress syndrome
- PRRs:
-
Pattern recognition receptors
- PAMPs:
-
Pathogen-associated molecular patterns
- TLRs:
-
Toll-like receptors
- CLRs:
-
C-type lectin receptors
- NLRs:
-
Nucleotide-binding oligomerization domain (NOD)-like receptors
- Th:
-
T-helper cells
- DC-SIGN:
-
Dendritic cell-specific intercellular adhesion molecule-3 grabbing nonintegrin
- MRs:
-
Mannose receptors
- IL:
-
Interleukin
- NLRP:
-
NLR family pyrin domain-containing proteins
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- RIG-1:
-
Retinoic acid inducible gene 1-like receptors
- CSS:
-
Cytokine storm syndrome
- CARD9:
-
Caspase recruitment domain-containing protein 9
- ALS:
-
Agglutinin-like sequence
- CFEM:
-
Common in several fungal extracellular membrane
- Hyr-1:
-
Hyphally regulated cell wall protein-1
- ROS:
-
Reactive oxygen spices
- HSP-90:
-
Heat shock protein-90
- Bcr-1:
-
Biofilm and cell wall regulator-1
- Efg-1:
-
Enhanced filamentous growth protein-1
- AFST:
-
Antifungal susceptibility testing
- ITS:
-
Internal transcribed spacer
- qPCR:
-
Quantitative polymerase chain reaction
- LDCs:
-
Least developed countries
- mAbs:
-
Monoclonal antibodies
- ACE-2:
-
Angiotensin converting enzyme
References
Amin, A., Vartanian, A., Poladian, N., Voloshko, A., Yegiazaryan, A., Al-Kassir, AL. Venketaraman, V. (2021) Root causes of fungal coinfections in COVID-19 infected patients. Infectious Disease Reports, 13.
Arastehfar, A., Carvalho, A., van de Veerdonk, FL., Jenks, JD., Koehler, P., Krause, R., Cornely, OAS. Perlin, D., Lass-Flörl, C. Hoenigl, M. (2020) COVID-19 associated pulmonary aspergillosis (CAPA)—From immunology to treatment. Journal of Fungi, 6.
Awada, B., Alam, W., Chalfoun, M., Araj, G., & Bizri, A. R. (2021). COVID-19 and Candida duobushaemulonii superinfection: A case report. Journal of Medical Mycology, 31, 101168.
Basso, R. P., Poester, V. R., Benelli, J. L., Stevens, D. A., Zogbi, H. E., Vasconcellos, ICd. S., Pasqualotto, A. C., & Xavier, M. O. (2021). COVID-19-associated histoplasmosis in an AIDS patient. Mycopathologia, 186, 109–112.
Bhatt, K., Agolli, A., Patel, MH., Garimella, R., Devi, M., Garcia, E., Amin, H., Domingue, C., Del Castillo, RG. Sanchez-Gonzalez, M. (2021) High mortality co-infections of COVID-19 patients: Mucormycosis and other fungal infections. Discoveries, 9.
Brown, G. D., Denning, D. W., Gow, N. A. R., Levitz, S. M., Netea, M. G., & White, T. C. (2012). Hidden killers: Human fungal infections. Science Translational Medicine, 4, 165rv113.
Catanzaro, M., Fagiani, F., Racchi, M., Corsini, E., Govoni, S., & Lanni, C. (2020). Immune response in COVID-19: Addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduction and Targeted Therapy, 5, 84.
Desai, J. V., Mitchell, A. P., & Andes, D. R. (2014). Fungal biofilms, drug resistance, and recurrent infection. Cold Spring Harbor Perspectives in Medicine, 4, a019729.
Drummond, R. A., Franco, L. M. L., & MS. (2018). Human CARD9: A critical molecule of fungal immune surveillance. Frontiers in Immunology, 9, 1836.
Dubourdeau, M., Athman, R., Balloy, V., Huerre, M., Chignard, M., Philpott, D. J., Latgé, J. P., & Ibrahim-Granet, O. (2006). Aspergillus fumigatus induces innate immune responses in alveolar macrophages through the MAPK pathway independently of TLR2 and TLR4. The Journal of Immunology, 177, 3994.
Gao, Q. Y., Chen, Y. X., & Fang, J. Y. (2020). 2019 novel coronavirus infection and gastrointestinal tract. Journal of digestive diseases, 21, 125.
Giollo, A., Adami, G., Gatti, D., Idolazzi, L., & Rossini, M. (2021). Coronavirus disease 19 (COVID-19) and non-steroidal anti-inflammatory drugs (NSAID). Annals of the Rheumatic Diseases, 80, e12.
Johnson, A. K., Ghazarian, Z., Cendrowski, K. D., & Persichino, J. G. (2021). Pulmonary aspergillosis and mucormycosis in a patient with COVID-19. Medical Mycology Case Reports, 32, 64–67.
Kowalski Caitlin, H., Morelli Kaesi, A., Schultz, D., Nadell Carey, D., & Cramer Robert, A. (2020). Fungal biofilm architecture produces hypoxic microenvironments that drive antifungal resistance. Proceedings of the National Academy of Sciences, 117, 22473–22483.
Kragholm, K., Torp-Pedersen, C., & Fosbol, E. (2021). Non-steroidal anti-inflammatory drug use in COVID-19. The Lancet Rheumatology, 3, e465–e466.
Lass-Flörl, C. (2017), in Human fungal pathogen identification: Methods and protocols, (Lion, T., ed.), Springer New York, New York, NY, pp. 3–15.
Laura Nunes, S., de ThaisPereira, M., de LíviaSouza, R., MartaHelena, B., Maryam, R., & dos Souza, SAndréLuis. (2020). Fungal infections in COVID-19-positive patients: A lack of optimal treatment options. Current Topics in Medicinal Chemistry, 20, 1951–1957.
Mang, S., Kaddu-Mulindwa, D., Metz, C., Becker, A., Seiler, F., Smola, S., Maßmann, A., Becker, S. L., Papan, C., Bals, R., Lepper, P. M., & Danziger, G. (2021). Pneumocystis jirovecii pneumonia and severe acute respiratory syndrome coronavirus 2 coinfection in a patient with newly diagnosed HIV-1 infection. Clinical Infectious Diseases, 72, 1487–1489.
Miyazato, A., Nakamura, K., Yamamoto, N., Mora-Montes Héctor, M., Tanaka, M., Abe, Y., Tanno, D., Inden, K., Gang, X., Ishii, K., Takeda, K., Akira, S., Saijo, S., Iwakura, Y., Adachi, Y., Ohno, N., Mitsutake, K., Gow Neil, A. R., Kaku, M., & Kawakami, K. (2009). Toll-like receptor 9-dependent activation of myeloid dendritic cells by deoxynucleic acids from Candida albicans. Infection and Immunity, 77, 3056–3064.
Moorthy, A., Gaikwad, R., Krishna, S., Hegde, R., Tripathi, K. K., Kale, P. G., Rao, P. S., Haldipur, D., & Bonanthaya, K. (2021). SARS-CoV-2, uncontrolled diabetes and corticosteroids—An unholy trinity in invasive fungal infections of the maxillofacial region? A retrospective, multi-centric analysis. Journal of Maxillofacial and Oral Surgery, 20, 418–425.
Nikoomanesh, F., Roudbarmohammadi, S., Khoobi, M., Haghighi, F., & Roudbary, M. (2019). Design and synthesis of mucoadhesive nanogel containing farnesol: Investigation of the effect on HWP1, SAP6 and Rim101 genes expression of Candida albicans in vitro. Artificial cells, nanomedicine, and biotechnology, 47, 64–72.
Nobile, C. J., & Mitchell, A. P. (2005). Regulation of cell-surface genes and biofilm formation by the C. albicans transcription factor Bcr1p. Current biology : CB, 15, 1150–1155.
Odamaki, T., Kato, K., Sugahara, H., Hashikura, N., Takahashi, S., Xiao, Jz., Abe, F., & Osawa, R. (2016). Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC microbiology, 16, 1–12.
Pathakumari, B., Liang, G., & Liu, W. (2020). Immune defence to invasive fungal infections: A comprehensive review. Biomedicine & Pharmacotherapy, 130, 110550.
Posteraro, B., Torelli, R., Vella, A., Leone, PM., De Angelis, G., De Carolis, E., Ventura, G., Sanguinetti, M. and Fantoni, M. (2020) Pan-echinocandin-resistant Candida glabrata bloodstream infection complicating COVID-19: A fatal case report. Jornal of Fungi, 6.
Ramage, G., Rajendran, R., Sherry, L., & Williams, C. (2012). Fungal biofilm resistance. International Journal of Microbiology, 2012, 528521.
Raut, A. H., & Huy, N. T. (2021). Rising incidence of mucormycosis in patients with COVID-19: Another challenge for India amidst the second wave? The Lancet Respiratory Medicine, S2213–2600(2221), 00265–264.
Ren, Z., Wang, H., Cui, G., Lu, H., Wang, L., Luo, H., Chen, X., Ren, H., Sun, R., Liu, W., Liu, X., Liu, C., Li, A., Wang, X., Rao, B., Yuan, C., Zhang, H., Sun, J., Chen, X., … Li, L. (2021). Alterations in the human oral and gut microbiomes and lipidomics in COVID-19. Gut, 70, 1253.
Rezasoltani, S., Yadegar, A., Hatami, B., Asadzadeh Aghdaei, H., & Zali, M. R. (2020). Antimicrobial resistance as a hidden menace lurking behind the COVID-19 outbreak: The global impacts of too much hygiene on AMR. Frontiers in microbiology, 11, 3097.
Robbins, N., Uppuluri, P., Nett, J., Rajendran, R., Ramage, G., Lopez-Ribot, J. L., & Cowen, L. E. A. D. (2011). Hsp90 governs dispersion and drug resistance of fungal biofilms. PLoS pathogens, 7, e1002257.
Romani, L. (2011). Immunity to fungal infections. Nature Reviews Immunology, 11, 275–288.
Root-Bernstein, R. (2021) Innate receptor activation patterns involving TLR and NLR synergisms in COVID-19, ALI/ARDS and sepsis cytokine storms: A review and model making novel predictions and therapeutic suggestions. Int J Mol Sci, 22.
Roudbary, M., Kumar, S., Kumar, A., Černáková, L., Nikoomanesh, F., & Rodrigues, C. F. (2021). Overview on the prevalence of fungal infections, immune response, and microbiome role in COVID-19 patients. Journal of Fungi, 7, 720.
Roudbary, M., Kumar, S., Kumar, A., Černáková, L., Nikoomanesh, F. and Rodrigues, C. F. (2021) Overview on the prevalence of fungal infections, immune response, and microbiome role in COVID-19 patients. Journal of Fungi, 7.
Santus, W., Devlin Jason, R., Behnsen, J. Ottemann Karen, M. (n.d) Crossing kingdoms: How the mycobiota and fungal-bacterial interactions impact host health and disease. Infection and Immunity, 89, e00648–00620.
Selarka, L., Sharma, A. K., Rathod, G., Saini, D., Patel, S., Sharma, V. K., & Lin, Y. L. (2021). Mucormycosis: A dreaded complication of COVID-19. QJM: An International Journal of Medicine, 114, 670–671.
Ventoulis, I., Sarmourli, T., Amoiridou, P., Mantzana, P., Exindari, M., Gioula, G. and Vyzantiadis, T.-A. (2020) Bloodstream infection by Saccharomyces cerevisiae in two COVID-19 patients after receiving supplementation of Saccharomyces in the ICU. Journal of Fungi, 6.
Von Borowski Rafael, G., Trentin Danielle, S. Johnson Karyn, N. Biofilms and Coronavirus reservoirs: (n.d) A perspective review. Applied and Environmental Microbiology, 87, e00859–00821.
Wang, J., Yang, Q., Zhang, P., Sheng, J., Zhou, J., & Qu, T. (2020). Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID-19 in Zhejiang, China: A retrospective case series. Critical Care, 24, 299.
White, P. L., Dhillon, R., Cordey, A., Hughes, H., Faggian, F., Soni, S., Pandey, M., Whitaker, H., May, A., Morgan, M., Wise, M. P., Healy, B., Blyth, I., Price, J. S., Vale, L., Posso, R., Kronda, J., Blackwood, A., Rafferty, H., … Backx, M. (2021). A national strategy to diagnose coronavirus disease 2019–associated invasive fungal disease in the intensive care unit. Clinical Infectious Diseases, 73, e1634–e1644.
Xu, K., Cai, H., Shen, Y., Ni, Q., Chen, Y., Hu, S., Li, J., Wang, H., Yu, L., & Huang, H. (2020). Management of COVID-19: The Zhejiang experience. Journal of Zhejiang University (medical science), 49, 147–157.
Yang, X., Yu, Y., Xu, J., Shu, H., Xia, Ja., Liu, H., Wu, Y., Zhang, L., Yu, Z., Fang, M., Yu, T., Wang, Y., Pan, S., Zou, X., Yuan, S., & Shang, Y. (2020). Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. The Lancet Respiratory Medicine, 8, 475–481.
Yasmin, F., Najeeb, H., Naeem, A., Dapke, K., Phadke, R., Asghar, M. S., Shah, S. M. I., De Berardis, D., & Ullah, I. (2021). COVID-19 associated mucormycosis: A systematic review from diagnostic challenges to management. Diseases, 9, 65.
Zuo, T., Wu, X., Wen, W. Lan, P. (2021) Gut microbiome alterations in COVID-19. Genomics, Proteomics & Bioinformatics.
Zuo, T., Zhan, H., Zhang, F., Liu, Q., Tso, E. Y. K., Lui, G. C. Y., Chen, N., Li, A., Lu, W., Chan, F. K. L., Chan, P. K. S., & Ng, S. C. (2020). Alterations in fecal fungal microbiome of patients with COVID-19 during time of hospitalization until discharge. Gastroenterology, 159, 1302-1310.e1305.
Funding
This work was supported by Brain Pool Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2021H1D3A2A01098535).
Author information
Authors and Affiliations
Contributions
Kumar Vishven Naveen: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft. Kandasamy Saravanakumar: conceptualization, data curation, formal analysis, investigation, methodology, writing—review and editing. Anbazhagan Sathiyaseelan: formal analysis, data curation, software. Davoodbasha MubarakAli: supervision, validation. Myeong-Hyeon Wang: project administration, resources, supervision, validation, writing—review and editing.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with animals or human participants.
Consent to Participate
The authors agreed to participate in this work.
Consent for Publication
The authors agreed to publish this work.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naveen, K.V., Saravanakumar, K., Sathiyaseelan, A. et al. Human Fungal Infection, Immune Response, and Clinical Challenge—a Perspective During COVID-19 Pandemic. Appl Biochem Biotechnol 194, 4244–4257 (2022). https://doi.org/10.1007/s12010-022-03979-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03979-5