Abstract
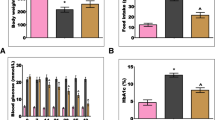
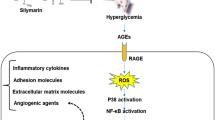
Diabetic retinopathy (DR) is the key cause of blindness and visual impairment in diabetes patients around the world. The high levels of oxidative stress in diabetes patients cause diabetic retinopathy. In addition to being an antioxidant, Bergenin also works as an immunosuppressant, an anti-inflammatory, and anticarcinogenic against hepatocarcinoma. This study examined the effects of Bergenin on diabetic retinopathy rats, using Streptozotocin (STZ) intraperitoneally to induce diabetes in rats. The animals were divided into four groups (n = 6), including a normal control (Group I), diabetic control (Group II), Bergenin (25 mg/kg) (Group III), and metformin (350 mg/kg) (Group IV). As previously mentioned, each animal received treatment for 60 days. To induce DR, rats were administered STZ (60 mg/kg) intraperitoneally for 60 days. Standard methods were utilized to measure the body weight of rats, blood glucose levels. We measured lipid profiles (Triglycerides, cholesterol, LDL, and HDL), inflammatory markers, and antioxidant levels with their respective kits. Analysis of retinal tissue morphometry and MMP-9, VEGF, and MCP-1 levels in serum was performed. Our research examined the expression levels of target genes (TNF-α, IL-1β, and IL-6) using RT-PCR analysis. STZ-induced animals that were treated with Bergenin had less food intake, lower blood glucose, and improved body weight. Bergenin significantly suppressed levels of pro-inflammatory cytokines, cholesterol, TG, LDL, AI, MMP-9, VEGF, and MCP-1 and increased the level of HDL and antioxidant enzymes in STZ-induced DR rats. As well as increasing antioxidant levels, reducing retinal thickness, and increasing cell numbers, Bergenin also lessened DR remarkably. The results of this study demonstrated that Bergenin effectively inhibited STZ-induced DR in rats.







Similar content being viewed by others
Data Availability
Data have been generated as part of the routine work.
References
Szarka, G., Balogh, M., Tengölics, Á. J., Ganczer, A., Völgyi, B., & Kovács-Öller, T. (2021). The role of gap junctions in cell death and neuromodulation in the retina. Neural Regeneration Research, 16(10), 1911–1920. https://doi.org/10.4103/1673-5374.308069
Altmann, C., & Schmidt, M. H. H. (2018). The role of microglia in diabetic retinopathy: Inflammation, microvasculature defects and neurodegeneration. International Journal of Molecular Sciences, 19(1), 110. https://doi.org/10.3390/ijms19010110
Kang, Q., & Yang, C. (2020). Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biology, 37, 101799. https://doi.org/10.1016/j.redox.2020.101799
Chiu, C. J., & Taylor, A. (2011). Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Progress in Retinal and Eye Research, 30(1), 18–53. https://doi.org/10.1016/j.preteyeres.2010.09.001
Yi, H., Xu, D., Wu, X., Xu, F., Lin, L., & Zhou, H. (2019). Isosteviol protects free fatty acid- and high fat diet-induced hepatic injury via modulating PKC-β/p66Shc/ROS and endoplasmic reticulum stress pathways. Antioxidants & Redox Signaling, 30(17), 1949–1968. https://doi.org/10.1089/ars.2018.7521
Shao, B., & Bayraktutan, U. (2014). Hyperglycaemia promotes human brain microvascular endothelial cell apoptosis via induction of protein kinase C-ßI and prooxidant enzyme NADPH oxidase. Redox Biology, 28(2), 694–701. https://doi.org/10.1016/j.redox.2014.05.005
Kany, S., Vollrath, J. T., & Relja, B. (2019). Cytokines in inflammatory disease. International journal of molecular sciences, 20(23), 6008. https://doi.org/10.3390/ijms20236008
Bajracharya, G. B. (2015). Diversity, pharmacology and synthesis of bergenin and its derivatives: Potential materials for therapeutic usages. Fitoterapia, 101, 133–152. https://doi.org/10.1016/j.fitote.2015.01.001
Patel, D., Patel, K., Kumar, R., Gadewar, M., & Tahilyani, V. (2012). Pharmacological and analytical aspects of bergenin: A concise report. Asian Pacific Journal of Tropical Disease, 2, 163–167.
Liu, J., Zhang, Y., Yu, C., Zhang, P., Gu, S., Wang, G., Xiao, H., & Li, S. (2021). Bergenin inhibits bladder cancer progression via activating the PPARγ/PTEN/Akt signal pathway. Drug Development Research, 82(2), 278–286. https://doi.org/10.1002/ddr.21751
Li, X., Wang, Y., Liang, J., Bi, Z., Ruan, H., Cui, Y., et al. (2021). Bergenin attenuates bleomycin-induced pulmonary fibrosis in mice via inhibiting TGF-β1 signaling pathway. Phytotherapy Research, 35(10), 5808–5822. https://doi.org/10.1002/ptr.7239
Sriset, Y., Chatuphonprasert, W., & Jarukamjorn, K. (2021). Bergenin attenuates sodium selenite-induced hepatotoxicity via improvement of hepatic oxidant-antioxidant balance in HepG2 cells and ICR mice. Journal of Biologically Active Products from Nature, 11(2), 97–115. https://doi.org/10.1080/22311866.2021.1908162
Bastikar, V.A., Bastikar, A., Gupta, P.P, Pai, S.R., Chhajed S.S. (2021) Target related in silico analysis of Bergenin and tuberculosis management. Biomedicine [Internet]. 2021Jan.1 [cited 2021Sep.15];40(4):474- 481. Available from: https://biomedicineonline.org/index.php/home/article/view/322
Zhang, G., Wang, H., Zhang, Q., Zhao, Z., Zhu, W., & Zuo, X. (2021). Bergenin alleviates H2 O2 -induced oxidative stress and apoptosis in nucleus pulposus cells: Involvement of the PPAR-γ/NF-κB pathway. Environmental Toxicology. https://doi.org/10.1002/tox.23368
Suryavanshi, S. V., & Kulkarni, Y. A. (2017). NF-κβ: A potential target in the management of vascular complications of diabetes. Frontiers in Pharmacology, 8, 798. https://doi.org/10.3389/fphar.2017.00798
Chen, Y., Sun, X. B., Lu, H. E., Wang, F., & Fan, X. H. (2017). Effect of luteoin in delaying cataract in STZ-induced diabetic rats. Archives of Pharmacal Research, 40(1), 88–95.
Xiao, D., Jin, K., Qiu, S., Lei, Q., Huang, W., Chen, H., Su, J., Xu, Q., Xu, Z., Gou, B., Tie, X., Liu, F., Liu, S., Liu, Y., & Xiang, M. (2021). In vivo regeneration of ganglion cells for vision restoration in mammalian retinas. Frontiers in Cell and Developmental Biology, 4(9), 755544. https://doi.org/10.3389/fcell.2021.755544
Lee, R., Wong, T. Y., & Sabanayagam, C. (2015). Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond)., 30(2), 17. https://doi.org/10.1186/s40662-015-0026-2
Wang, W., & Lo, A. C. Y. (2018). Diabetic retinopathy: Pathophysiology and treatments. International Journal of Molecular Sciences, 19(6), 1816. https://doi.org/10.3390/ijms19061816
Wong, T. Y., Cheung, C. M., Larsen, M., Sharma, S., & Simo, R. (2016). Diabetic retinopathy. Nature Reviews Disease Primers, 2, 16012.
Chawla, A., Chawla, R., & Jaggi, S. (2016). Microvascular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J Endocrinol Metab., 20(4), 546–551.
AlSharari, S. D., Al-Rejaie, S. S., Abuohashish, H. M., Aleisa, A. M., Parmar, M. Y., & Mohammed, M. A. (2014). Ameliorative potential of morin in streptozotocin-induced neuropathic pain in rats. Tropical Journal of Pharmaceutical Research, 13(9), 1429–1436.
Forrester, J. V., Kuffova, L., & Delibegovic, M. (2020). The role of inflammation in diabetic retinopathy. Frontiers in Immunology, 11, 583687. https://doi.org/10.3389/fimmu.2020.583687
Koleva-Georgieva, D. N., Sivkova, N. P., & Terzieva, D. (2011). Serum inflammatory cytokines IL-1beta, IL-6, TNF-alpha and VEGF have influence on the development of diabetic retinopathy. Folia Med (Plovdiv), 53(2), 44–50. https://doi.org/10.2478/v10153-010-0036-8
Doganay, S., Evereklioglu, C., Er, H., et al. (2002). Comparison of serum NO, TNF-α, IL-1β, sIL-2R, IL-6 and IL-8 levels with grades of retinopathy in patients with diabetes mellitus. Eye, 16, 163–170. https://doi.org/10.1038/sj.eye.6700095
Huang, H., Gandhi, J. K., Zhong, X., Wei, Y., Gong, J., Duh, E. J., Vinores, S. A. (2011). TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Investigative Ophthalmology & Visual Science, 52(3), 1336–44. https://doi.org/10.1167/iovs.10-5768
Meleth, A. D., Agrón, E., Chan, C. C., Reed, G. F., Arora, K., Byrnes, G., Csaky, K. G., Ferris, F. L., 3rd., & Chew, E. Y. (2005). Serum inflammatory markers in diabetic retinopathy. Investigative Ophthalmology & Visual Science, 46(11), 4295–4301. https://doi.org/10.1167/iovs.04-1057
Shibuya, M. (2011). Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes & Cancer, 2(12), 1097–1105. https://doi.org/10.1177/1947601911423031
Kota, S. K., Meher, L. K., Jammula, S., Kota, S. K., Krishna, S. V., et al. (2012). Aberrant angiogenesis: The gateway to diabetic complications. Ind. J. Endo. Metabol., 16(6), 918.
N.M., Beaulieu, W.T., Bressler, S.B., Glassman, A.R., Melia, B.M., Jampol, L. M., Jhaveri, C.D., Salehi-Had, H., Velez, G., Sun, J.K., DRCR Retina Network. (2020). Anti-vascular endothelial growth factor therapy and risk of traction retinal detachment in eyes with proliferative diabetic retinopathy: Pooled analysis of five DRCR retina network randomized clinical trials. Retina. 40(6), 1021-1028
Titchenell, P. M., & Antonetti, D. A. (2013). Using the past to inform the future: Anti-VEGF therapy as a road map to develop novel therapies for diabetic retinopathy. Diabetes, 62, 1808–1815.
Treins, C., Giorgetti-Peraldi, S., Murdaca, J., & Van Obberghen, E. (2001). Regulation of vascular endothelial growth factor expression by advanced glycation end products. Journal of Biological Chemistry, 276, 43836–43841.
Aiello, L. P., Bursell, S. E., Clermont, A., Duh, E., Ishii, H., Takagi, C., Mori, F., Ciulla, T. A., Ways, K., Jirousek, M., Smith, L. E., & King, G. L. (1997). Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes., 46, 10473-10480.a.
Waszczykowska, A., Podgórski, M., Waszczykowski, M., Gerlicz-Kowalczuk, Z., & Jurowski, P. (2020). Matrix metalloproteinases MMP-2 and MMP-9, their inhibitors TIMP-1 and TIMP-2, vascular endothelial growth factor and sVEGFR-2 as predictive markers of ischemic retinopathy in patients with systemic sclerosis-case series report. International Journal of Molecular Sciences, 21(22), 8703. https://doi.org/10.3390/ijms21228703
Solanki, A., Bhatt, L. K., Johnston, T. P., Prabhavalkar, K. S. (2019). Targeting matrix metalloproteinases for diabetic retinopathy: The way ahead? Current Protein & Peptide Science, 20(4), 324–333. https://doi.org/10.2174/1389203719666180914093109
Kowluru, R. A., Shan, Y., & Mishra, M. (2016). Dynamic DNA methylation of matrix metalloproteinase-9 in the development of diabetic retinopathy. Laboratory Investigation, 96(10), 1040–1049.
Lima-Fontes, M., Barata, P., Falcão, M., & Carneiro, Â. (2020). Ocular findings in metabolic syndrome: A review. Porto Biomed J., 5(6), e104. https://doi.org/10.1097/j.pbj.0000000000000104
Crane, Isabel J., & Liversidge, Janet. (2008). Mechanisms of leukocyte migration across the blood-retina barrier. Seminars in immunopathology, 30,2(2008), 165–77. https://doi.org/10.1007/s00281-008-0106-7
Huang, H., Gandhi, J. K., Zhong, X., Wei, Y., Gong, J., Duh, E. J., & Vinores, S. A. (2011). TNF alpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Investigative Ophthalmology & Visual Science, 52(3), 1336–1344.
Author information
Authors and Affiliations
Contributions
All authors contributed equally.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
All authors has their consent to participate.
Consent for Publication
All authors has their consent to publish their work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yin, Y., Xu, R., Ning, L. et al. Bergenin alleviates Diabetic Retinopathy in STZ-induced rats. Appl Biochem Biotechnol 195, 5299–5311 (2023). https://doi.org/10.1007/s12010-022-03949-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03949-x