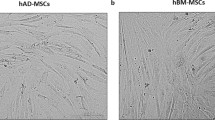
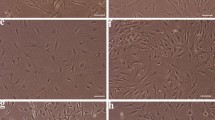
Abstract
In recent years, stem cell therapy has shown promise in regenerative medicine. The lack of standardized protocols for cell isolation and differentiation generates conflicting results in this field. Mesenchymal stem cells derived from adipose tissue (ASC) and fibroblasts (FIB) share very similar cell membrane markers. In this context, the distinction of mesenchymal stem cells from fibroblasts has been crucial for safe clinical application of these cells. In the present study, we developed aptamers capable of specifically recognize ASC using the Cell-SELEX technique. We tested the affinity of ASC aptamers compared to dermal FIB. Quantitative PCR was advantageous for the in vitro validation of four candidate aptamers. The binding capabilities of Apta 2 and Apta 42 could not distinguish both cell types. At the same time, Apta 21 and Apta 99 showed a better binding capacity to ASC with dissociation constants (Kd) of 50.46 ± 2.28 nM and 72.71 ± 10.3 nM, respectively. However, Apta 21 showed a Kd of 86.78 ± 9.14 nM when incubated with FIB. Therefore, only Apta 99 showed specificity to detect ASC by total internal reflection microscopy (TIRF). This aptamer is a promising tool for the in vitro identification of ASC. These results will help understand the differences between these two cell types for more specific and precise cell therapies.




Similar content being viewed by others
Data availability
The data that supports the findings of this study are included in this article and available in the supplementary information.
Change history
07 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s12010-021-03646-1
References
Sefah, K., Shangguan, D., Xiong, X., O'Donoghue, M. B., & Tan, W. (2010). Development of DNA aptamers using Cell-SELEX. Nature protocols, 5(6), 1169–1185. https://doi.org/10.1038/nprot.2010.66.
Tuerk, C., & Gold, L. (1990). Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science (New York, N.Y.), 249(4968), 505–510. https://doi.org/10.1126/science.2200121.
Ellington, A. D., & Szostak, J. W. (1990). In vitro selection of RNA molecules that bind specific ligands. Nature, 346(6287), 818–822. https://doi.org/10.1038/346818a0.
Quang, N. N., Miodek, A., Cibiel, A., & Ducongé, F. (2017). Selection of aptamers against whole living cells: From Cell-SELEX to identification of biomarkers. Methods in molecular biology (Clifton, N.J.), 1575, 253–272. https://doi.org/10.1007/978-1-4939-6857-216.
Zuk, P. A., Zhu, M., Ashjian, P., De Ugarte, D. A., Huang, J. I., Mizuno, H., Alfonso, Z. C., Fraser, J. K., Benhaim, P., & Hedrick, M. H. (2002). Human adipose tissue is a source of multipotent stem cells. Molecular biology of the cell, 13(12), 4279–4295. https://doi.org/10.1091/mbc.e02-02-0105.
Zuk, P. (2013). Adipose-Derived Stem Cells in Tissue Regeneration: A Review. Int Sch Res Not, 2013, e713959–e713935. https://doi.org/10.1155/2013/713959.
Pereira, M. C., Secco, M., Suzuki, D. E., Janjoppi, L., Rodini, C. O., Torres, L. B., Araújo, B. H., Cavalheiro, E. A., Zatz, M., & Okamoto, O. K. (2011). Contamination of mesenchymal stem-cells with fibroblasts accelerates neurodegeneration in an experimental model of Parkinson's disease. Stem cell reviews and reports, 7(4), 1006–1017. https://doi.org/10.1007/s12015-011-9256-4.
Halfon, S., Abramov, N., Grinblat, B., & Ginis, I. (2011). Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem cells and development, 20(1), 53–66. https://doi.org/10.1089/scd.2010.0040.
Kundrotas, G. (2012). Surface markers distinguishing mesenchymal stem cells from fibroblastos. Acta medica Lituanica, 19(2), 75–79. https://doi.org/10.6001/actamedica.v19i2.2313.
Bourin, P., Bunnell, B. A., Casteilla, L., Dominici, M., Katz, A. J., March, K. L., Redl, H., Rubin, J. P., Yoshimura, K., & Gimble, J. M. (2013). Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy, 15(6), 641–648. https://doi.org/10.1016/j.jcyt.2013.02.006.
Flavell, S. J., Hou, T. Z., Lax, S., Filer, A. D., Salmon, M., & Buckley, C. D. (2008). Fibroblasts as novel therapeutic targets in chronic inflammation. British journal of pharmacology, 153 Suppl, 1(Suppl 1), S241–S246. https://doi.org/10.1038/sj.bjp.0707487.
Soundararajan, M., & Kannan, S. (2018). Fibroblasts and mesenchymal stem cells: Two sides of the same coin? Journal of cellular physiology, 233(12), 9099–9109. https://doi.org/10.1002/jcp.26860.
Alt, E., Yan, Y., Gehmert, S., Song, Y. H., Altman, A., Gehmert, S., Vykoukal, D., & Bai, X. (2011). Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biology of the cell, 103(4), 197–208. https://doi.org/10.1042/BC20100117.
Denu, R. A., Nemcek, S., Bloom, D. D., Goodrich, A. D., Kim, J., Mosher, D. F., & Hematti, P. (2016). Fibroblasts and mesenchymal stromal/stem cells are phenotypically indistinguishable. Acta haematologica, 136(2), 85–97. https://doi.org/10.1159/000445096.
Ardjomandi, N., Niederlaender, J., Aicher, W. K., Reinert, S., Schweizer, E., Wendel, H. P., & Alexander, D. (2013). Identification of an aptamer binding to human osteogenic-induced progenitor cells. Nucleic acid therapeutics, 23(1), 44–61. https://doi.org/10.1089/nat.2012.0349.
Ueki, R., Atsuta, S., Ueki, A., Hoshiyama, J., Li, J., Hayashi, Y., & Sando, S. (2019). DNA aptamer assemblies as fibroblast growth factor mimics and their application in stem cell culture. Chemical communications (Cambridge, England), 55(18), 2672–2675. https://doi.org/10.1039/c8cc08080a.
Hou, Z., Meyer, S., Propson, N. E., Nie, J., Jiang, P., Stewart, R., & Thomson, J. A. (2015). Characterization and target identification of a DNA aptamer that labels pluripotent stem cells. Cell research, 25(3), 390–393. https://doi.org/10.1038/cr.2015.7.
Wang, M., Wu, H., Li, Q., Yang, Y., Che, F., Wang, G., & Zhang, L. (2019). Novel aptamer-functionalized nanoparticles enhances bone defect repair by improving stem cell recruitment. International journal of nanomedicine, 14, 8707–8724. https://doi.org/10.2147/IJN.S223164.
Wang, X., Song, X., Li, T., Chen, J., Cheng, G., Yang, L., & Chen, C. (2019). Aptamer-functionalized bioscaffold enhances cartilage repair by improving stem cell recruitment in osteochondral defects of rabbit knees. The American Journal of Sports Medicine, 47(10), 2316–2326. https://doi.org/10.1177/0363546519856355.
Abreu de Melo, M. I., da Silva Cunha, P., Coutinho de Miranda, M., Faraco, C., Barbosa, J. L., da Fonseca Ferreira, A., Kunrath Lima, M., Faria, J., Rodrigues, M. Â., de Goes, A. M., & Gomes, D. A. (2021). Human adipose-derived stromal/stem cells are distinct from dermal fibroblasts as evaluated by biological characterization and RNA sequencing. Cell biochemistry and function. https://doi.org/10.1002/cbf.3610.Advanceonlinepublication.
Graziani, A. C., Stets, M. I., Lopes, A. L. K., Stets, M. I., Lopes, A. L. K., Schluga, P. H. C., Marton, S., Mendes, I. F., Andrade, A. S. R. ., Krieger, M. A., & Cardoso, J. (2017). High efficiency binding aptamers for a wide range of bacterial sepsis agents. J Microbiol Biotechnol, 27(4), 838–843. https://doi.org/10.4014/jmb.1611.11004.
Thiel, W. H., Bair, T., Peek, A. S., Liu, X., Dassie, J., Stockdale, K. R., Behlke, M. A., Miller Jr., F. J., & Giangrande, P. H. (2012). Rapid identification of cell-specific, internalizing RNA aptamers with bioinformatics analyses of a cell-based aptamer selection. PloS one, 7(9), e43836. https://doi.org/10.1371/journal.pone.0043836.
Mencin, N., Šmuc, T., Vraničar, M., Mavri, J., Hren, M., Galeša, K., Krkoč, P., Ulrich, H., & Šolar, B. (2014). Optimization of SELEX: Comparison of different methods for monitoring the progress of in vitro selection of aptamers. Journal of pharmaceutical and biomedical analysis, 91, 151–159. https://doi.org/10.1016/j.jpba.2013.12.031.
Thiel, W. H. (2016). Galaxy workflows for web-based bioinformatics analysis of aptamer high-throughput sequencing data. Molecular therapy. Nucleic acids, 5(8), e345. https://doi.org/10.1038/mtna.2016.54.
Saitou, N., & Nei, M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular biology and evolution, 4(4), 406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular biology and evolution, 35(6), 1547–1549. https://doi.org/10.1093/molbev/msy096.
Liu, W., & Saint, D. A. (2002). A new quantitative method of real time reverse transcription polymerase chain reaction assay based on simulation of polymerase chain reaction kinetics. Analytical biochemistry, 302(1), 52–59. https://doi.org/10.1006/abio.2001.5530.
Cikos, S., Bukovská, A., & Koppel, J. (2007). Relative quantification of mRNA: comparison of methods currently used for real-time PCR data analysis. BMC molecular biology, 8(1), 113. https://doi.org/10.1186/1471-2199-8-113.
Pfaffl, M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic acids research, 29(9), 45e–445e. https://doi.org/10.1093/nar/29.9.e45.
Mariane Izabella Abreu de Melo., Rodrigues Correa, C., da Silva Cunha, P., Alfredo Miranda de Góes., Assis Gomes, D., & Antero Silva Ribeiro de Andrade. (2020). DNA aptamers selection for carcinoembryonic antigen (CEA). Bioorganic & medicinal chemistry letters, 30(15), 127278. https://doi.org/10.1016/j.bmcl.2020.127278
Souza, A. G., Marangoni, K., Fujimura, P. T., Alves, P. T., Silva, M. J., Bastos, V. A., Goulart, L. R., & Goulart, V. A. (2016). 3D Cell-SELEX: Development of RNA aptamers as molecular probes for PC-3 tumor cell line. Experimental cell research, 341(2), 147–156. https://doi.org/10.1016/j.yexcr.2016.01.015.
Vidic, M., Smuc, T., Janez, N., Blank, M., Accetto, T., Mavri, J., Nascimento, I. C., Nery, A. A., Ulrich, H., & Lah, T. T. (2018). In silico selection approach to develop DNA aptamers for a stem-like cell subpopulation of non-small lung cancer adenocarcinoma cell line A549. Radiology and oncology, 52(2), 152–159. https://doi.org/10.2478/raon-2018-0014.
Nascimento, I. C., Nery, A. A., Bassaneze, V., Krieger, J. E., & Ulrich, H. (2016). Applications of aptamers in flow and imaging cytometry. Methods in molecular biology (Clifton, N.J.), 1380, 127–134. https://doi.org/10.1007/978-1-4939-3197-210.
Avci-Adali, M., Wilhelm, N., Perle, N., Stoll, H., Schlensak, C., & Wendel, H. P. (2013). Absolute quantification of cell-bound DNA aptamers during SELEX. Nucleic acid therapeutics, 23(2), 125–130. https://doi.org/10.1089/nat.2012.0406.
Li, H. H., Wen, C. Y., Hong, C. Y., & Lai, J. C. (2017). Evaluation of aptamer specificity with or without primers using clinical samples for C-reactive protein by magnetic-assisted rapid aptamer selection. RSC Adv, 7(68), 42856–42865. https://doi.org/10.1039/c7ra07249j.
Fish, K. N. (2009). Total internal reflection fluorescence (TIRF) microscopy. Current protocols in cytometry, Chapter 12, Unit12.18, 50(1). https://doi.org/10.1002/0471142956.cy1218s50.
Funatsu, T., Harada, Y., Tokunaga, M., Saito, K., & Yanagida, T. (1995). Imaging of single fluorescent molecules and individual ATP turnovers by single myosin molecules in aqueous solution. Nature, 374(6522), 555–559. https://doi.org/10.1038/374555a0.
Liu, K., Lin, B., & Lan, X. (2013). Aptamers: A promising tool for cancer imaging, diagnosis, and therapy. Journal of cellular biochemistry, 114(2), 250–255. https://doi.org/10.1002/jcb.24373.
Berezovski, M. V., Lechmann, M., Musheev, M. U., Mak, T. W., & Krylov, S. N. (2008). Aptamer-facilitated biomarker discovery (AptaBiD). Journal of the American Chemical Society, 130(28), 9137–9143. https://doi.org/10.1021/ja801951p.
Acknowledgements
The authors kindly acknowledge GenXPro GmbH (Frankfurt am Main, Germany) for their assistance in aptamers sequencing. The authors also thank FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for funding.
Funding
This study was funded by FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Mariane Izabella Abreu de Melo, Pricila da Silva Cunha, Marcelo Coutinho de Miranda, Joana Lobato Barbosa, Jerusa Araújo Quintão Arantes Faria, and Dawidson Assis Gomes. Funding acquisition, resources, and supervision were provided by Michele Angela Rodrigues, Pricila da Silva Cunha, Alfredo Miranda de Goes, and Dawidson Assis Gomes. Mariane Izabella Abreu de Melo wrote the first draft of the manuscript, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Research Ethics Committee of the Universidade Federal de Minas Gerais (No. 02508018.1.0000.5149).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article unfortunately contained a mistake in the supplementary files and figure 1. The supplementary files and figure 1 has been corrected.
Supplementary Information
Fig. S1
Determination of the optimal number of PCR cycles in the positive selection. The figure shows electrophoresis images of the amplicons produced by PCR in the positive selection cycle tests. Agarose gel (2%) was stained with ethidium bromide. The order in the gel is: (M): Step-Ladder marker 50 bp, (12) to (20): amplicons produced from 12,14,16, 18 and 20 cycles of the PCR reaction, respectively, (NTC):no-template control. Bp= base pair. (A) 3rd round, (B) 4th round, (C) 5th round, (D) 6th round, (E) 7th round and (F) 8th round (PNG 24663 kb)
Fig. S2
Determination of the optimal number of PCR cycles in the negative selection. The figure shows electrophoresis images of the amplicons produced by PCR in the negative selection cycle tests. Agarose gel (2%) was stained with ethidium bromide. The order in the gel is: (M): Step-Ladder marker 50 bp, (16) to (22): amplicons produced from 16, 18, 20 and 22 cycles of the PCR reaction, respectively, (NTC):no-template control. Bp= base pair. (A) 3rd round, (B) 4th round, (C) 5th round, (D) 6th round, (E) 7th round and (F) 8th round. (PNG 24663 kb)
Fig. S3
Melting profiles of aptamers in all selection rounds. (a) Melt curve of aptamers from the library and round 1 (b) Melt curve of aptamers from rounds 1 and 2. (c) Melt curve of aptamers from rounds 2 and 3. (d) Melt curve of aptamers from rounds 3 and 4. (e) Melt curve of aptamers from rounds 4 and 5. (f) Melt curve of aptamers from rounds 5 and 6. (g) Melt curve of aptamers from rounds 6 and 7. (h) Melt curve of aptamers from rounds 7 and 8. (i) Melt curve from the library and round 8. X-axis: temperature (°C); Y-axis: Derivative reporter (-Rn); NTC (no-template control). The graphs of fluorescence intensity versus temperature were generated on the 7.500 Software v. 2.0.6 (Applied Biosystems) (PNG 11591 kb)
Fig. S4
Apta 99 shows a higher binding capacity to ASC compared to FIB. Total internal reflection microscopy (TIRF) was used to investigate the ability of the aptamers to bind at the regions of the plasma membrane of the cells. ASC and FIB were incubated for 30 min with 250 nm of aptamers at 37°C. Next, cells were washed and observed using a Leica TIRF infinity high power microscope (Leica Microsystems, Germany) with a 100x objective, N.A. 1.47. (a) The representative images show that the fluorescence of Apta 99 was higher in ASC compared to FIB. (b) The average quantification of the fluorescence intensity of the TIRF images confirms that Apta 99 can bind more specifically in ASC (429,7 ± 69,84) than in FIB (118 ± 3,786). (c) The representative images show that the fluorescence of Apta 21 was similar between ASC and FIB. (d) The average quantification of the fluorescence intensity of the TIRF images does not show any difference between ASC (314,7 ± 142,6) and FIB (371 ± 82,87). Unpaired t-test with Welch’s correction; n=3; * p<0.05. Scale bar = 10 μm (PNG 23212 kb)
Rights and permissions
About this article
Cite this article
de Melo, M.I.A., da Silva Cunha, P., de Miranda, M.C. et al. Selection of DNA Aptamers for Differentiation of Human Adipose-Derived Mesenchymal Stem Cells from Fibroblasts. Appl Biochem Biotechnol 193, 3704–3718 (2021). https://doi.org/10.1007/s12010-021-03618-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03618-5