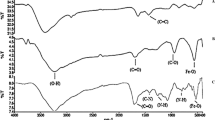
Abstract
The production of microbial lipid using lignocellulosic agroforestry residues has attracted much attention. But, various inhibitors such as phenols and furans, which are produced during lignocellulosic hydrolysate preparation, are harmful to microbial lipid accumulation. Herein, we developed a novel detoxification strategy of rice straw hydrolysate using immobilized laccase on magnetic Fe3O4 nanoparticles for improving lipid production of Rhodotorula glutinis. Compared with free laccase, the immobilized laccase on magnetic nanoparticles showed better stability, which still retained 76% of original activity at 70 °C and 56% at pH 2 for 6 h. This immobilized laccase was reused to remove inhibitors in acid-pretreated rice straw hydrolysate through recycling with external magnetic field. The results showed that most of phenols, parts of furans, and formic acids could be removed by immobilized laccase after the first batch. Notably, the immobilized laccase exhibited good reusability in repeated batch detoxification. 78.2% phenols, 43.8% furfural, 30.4% HMF, and 16.5% formic acid in the hydrolysate were removed after the fourth batch. Furthermore, these detoxified rice straw hydrolysates, as substrates, were applied to the lipid production of Rhodotorula glutinis. The lipid yield in detoxified hydrolysate was significantly higher than that in undetoxified hydrolysate. These findings suggest that the immobilized laccase on magnetic nanoparticles has a potential to detoxify lignocellusic hydrolysate for improving microbial lipid production.







Similar content being viewed by others
References
Alonso, D. M., Bond, J. Q., & Dumesic, J. A. (2010). Catalytic conversion of biomass to biofuels. Green Chemistry, 12(9), 1493–1513.
Huang, C., Wu, H., & Liu, Q. P. (2011). Effects of aldehydes on the growth and lipid accumulation of oleaginous yeast Trichosporon fermentans. Journal of Agricultural and Food Chemistry, 59(9), 4606–4613.
Jin, M., Slininger, P. J., Dien, B. S., Waghmode, S., & Balan, V. (2014). Microbial lipid-based lignocellulosic biorefinery: Feasibility and challenges. Trends in Biotechnology, 33(1), 43–54.
Annamalai, N., Sivakumar, N., & Oleskowicz-Popiel, P. (2018). Enhanced production of microbial lipids from waste office paper by the oleaginous yeast Cryptococcus curvatus. Fuel, 217, 420–426.
Tang, B., Lei, P., Xu, Z. Q., Jiang, Y. X., Xu, Z., Liang, J. F., Feng, X. H., & Xu, H. (2015). Highly efficient rice straw utilization for poly-(γ-glutamic acid) production by Bacillus subtilis NX-2. Bioresource Technology, 193, 370–376.
Kumar, P., Barrett, D. M., Delwiche, M. J., & Stroeve, P. (2009). Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Industrial & Engineering Chemistry Research, 48(8), 3713–3729.
Kumar, R., Singh, S., & Singh, O. V. (2008). Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives. Journal of Industrial Microbiology & Biotechnology, 35(5), 377–391.
Xie, L., Zhao, J., Wu, J., Gao, M., Zhao, Z., Lei, X., Zhao, Y., Yang, W., Gao, X., Ma, C., Liu, H., Wu, F., Wang, X., Zhang, F., Guo, P., & Dai, G. (2015). Efficient hydrolysis of corncob residue through cellulolytic enzymes from Trichoderma strain G26 and L-lactic acid preparation with the hydrolysate. Bioresource Technology, 193, 331–336.
Pol, E. C. V. D., Bakker, R. R., Baets, P., & Eggink, G. (2014). By-products resulting from lignocellulose pretreatment and their inhibitory effect on fermentations for (bio)chemicals and fuels. Applied Microbiology and Biotechnology, 98(23), 9579–9593.
Jonsson, L. J., Alriksson, B., & Nilvebrant, N.-O. (2013). Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnology for Biofuels, 6, 16.
Mussatto, S. I., & Roberto, I. C. (2004). Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: a review. Bioresource Technology, 93(1), 1–10.
Nguyen, N., Fargues, C., Guiga, W., & Lameloise, M. L. (2015). Assessing nanofiltration and reverse osmosis for the detoxification of lignocellulosic hydrolysates. Journal of Membrane Science, 487, 40–50.
Soudham, V. P., Brandberg, T., Mikkola, J. P., & Larsson, C. (2014). Detoxification of acid pretreated spruce hydrolysates with ferrous sulfate and hydrogen peroxide improves enzymatic hydrolysis and fermentation. Bioresource Technology, 166, 559–565.
Kapoor, R. K., Rajan, K., & Carrier, D. J. (2015). Applications of Trametes versicolor crude culture filtrates in detoxification of biomass pretreatment hydrolyzates. Bioresource Technology, 189, 99–106.
Zhang, D., Ong, Y. L., Li, Z., & Wu, J. C. (2013). Biological detoxification of furfural and 5-hydroxyl methyl furfural in hydrolysate of oil palm empty fruit bunch by Enterobacter sp. FDS8. Biochemical Engineering Journal, 72, 77–82.
Okuda, N., Soneura, M., Ninomiya, K., Katakura, Y., & Shioya, S. (2008). Biological detoxification of waste house wood hydrolysate using Ureibacillus thermosphaericus for bioethanol production. Journal of Bioscience and Bioengineering, 106(2), 128–133.
Morsi, R., Bilal, M., Iqbal, H., & M, N., Ashraf, S, S. (2020). Laccases and peroxidases: the smart, greener and futuristic biocatalytic tools to mitigate recalcitrant emerging pollutants. Science of the Total Environment, 714, 136572. https://doi.org/10.1016/j.scitotenv.2020.136572.
Bilal, M., Iqbal, H. M. N., & Barceló, D. (2019). Mitigation of bisphenol A using an array of laccase-based robust bio-catalytic cues – a review. Science of the Total Environment, 689, 160–177.
Moreno, A. D., Ibarra, D., Fernández, J. L., & Ballesteros, M. (2012). Different laccase detoxification strategies for ethanol production from lignocellulosic biomass by the thermotolerant yeast Kluyveromyces marxianus CECT 10875. Bioresource Technology, 106, 101–109.
Chandel, A. K., Kapoor, R. K., Singh, A., & Kuhad, R. C. (2007). Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM 3501. Bioresource Technology, 98(10), 1947–1950.
Ren, S., Li, C., Jiao, X., Jia, S., Jiang, Y., Bilal, M., & Cui, J. (2019). Recent progress in multienzymes co-immobilization and multienzyme system applications. Chemical Engineering Journal, 373, 1254–1278.
Bezerra, T. M. D. S., Bassan, J. C., Santos, V. T. D. O., Ferraz, A., & Monti, R. (2015). Covalent immobilization of laccase in green coconut fiber and use in clarification of apple juice. Process Biochemistry, 50(3), 417–423.
Fernández-Fernández, M., Sanromán, M. Á., & Moldes, D. (2013). Recent developments and applications of immobilized laccase. Biotechnology Advances, 31(8), 1808–1825.
Xu, R., Chi, C., Li, F., & Zhang, B. (2013). Laccase–polyacrylonitrile nanofibrous membrane: highly immobilized, stable, reusable, and efficacious for 2,4,6-trichlorophenol removal. ACS Appled. Materials & Interfaces, 5(23), 12554–12560.
Pang, R., Li, M., & Zhang, C. (2015). Degradation of phenolic compounds by laccase immobilized on carbon nanomaterials: diffusional limitation investigation. Talanta, 131, 38–45.
Bilal, M., Anh Nguyen, T., & Iqbal, H. M. N. (2020). Multifunctional carbon nanotubes and their derived nano-constructs for enzyme immobilization – a paradigm shift in biocatalyst design. Coordination Chemistry Reviews, 422, 213475. https://doi.org/10.1016/j.ccr.2020.213475.
Shi, L., Ma, F., Han, Y., Zhang, X., & Yu, H. (2014). Removal of sulfonamide antibiotics by oriented immobilized laccase on Fe3O4 nanoparticles with natural mediators. Journal of Hazardous Materials, 279, 203–211.
Wang, H., Wei, Z., Zhao, J., Xu, L., Zhou, C., Lin, C., & Wang, L. (2013). Rapid decolorization of phenolic azo dyes by immobilized laccase with Fe3O4/SiO2 nanoparticles as support. Industrial & Engineering Chemistry Research, 52(12), 4401–4407.
Das, A., Singh, J., & Yogalakshmi, K. N. (2017). Laccase immobilized magnetic iron nanoparticles: Fabrication and its performance evaluation in chlorpyrifos degradation. International Biodeterioration & Biodegradation, 117, 183–189.
Xia, T. T., Liu, C. Z., Hu, J. H., & Guo, C. (2016). Improved performance of immobilized laccase on amine-functioned magnetic Fe3O4 nanoparticles modified with polyethylenimine. Chemical Engineering Journal, 295, 201–206.
Yin, L., Ye, J. Y., Kuang, S. B., Guan, Y. Q., & You, R. (2017). Induction, purification, and characterization of a thermo and pH stable laccase from Abortiporus biennis J2 and its application on the clarification of litchi juice. Bioscience, Biotechnology, and Biochemistry, 81(5), 1033–1040.
Lee, K. M., Kalyani, D., Tiwari, M. K., Kim, T. S., Dhiman, S. S., Lee, J. K., & Kim, I. W. (2012). Enhanced enzymatic hydrolysis of rice straw by removal of phenolic compounds using a novel laccase from yeast Yarrowia lipolytica. Bioresource Technology, 123, 636–645.
Fu, L., Cui, X., Li, Y., Xu, L., Zhang, C., Xiong, R., Zhou, D., & Crittenden, J. C. (2017). Excessive phosphorus enhances Chlorella regularis lipid production under nitrogen starvation stress during glucose heterotrophic cultivation. Chemical Engineering Journal, 330, 566–572.
Gao, R., Hao, Y., Zhang, L., Cui, X., Liu, D., Zhang, M., Tang, Y., & Zheng, Y. (2016). A facile method for protein imprinting on directly carboxyl-functionalized magnetic nanoparticles using non-covalent template immobilization strategy. Chemical Engineering Journal, 284, 139–148.
Verma, M. L., Chaudhary, R., Tsuzuki, T., Barrow, C. J., & Puri, M. (2013). Immobilization of β-glucosidase on a magnetic nanoparticle improves thermostability: application in cellobiose hydrolysis. Bioresource Technology, 135, 2–6.
Chen, G., Ma, Y., Su, P., & Fang, B. (2012). Direct binding glucoamylase onto carboxyl- functioned magnetic nanoparticles. Biochemical Engineering Journal, 67, 120–125.
Zhu, Y. T., Ren, X. Y., Liu, Y. M., Wei, Y., Qing, L. S., & Liao, X. (2014). Covalent immobilization of porcine pancreatic lipase on carboxyl-activated magnetic nanoparticles: Characterization and application for enzymatic inhibition assays. Materials Science & Engineering, C: Materials for Biological Applications, 38, 278–285.
Xiao, A., Xiao, Q., Lin, Y., Ni, H., Zhu, Y., & Cai, H. (2016). Immobilization of agarase from marine vibrio onto carboxyl-functioned magnetic nanoparticles. Journal of Nanoscience and Nanotechnology, 16(9), 10048–10055.
Moreno, A. D., Ibarra, D., Ballesteros, I., Fernández, J. L., & Ballesteros, M. (2013). Ethanol from laccase-detoxified lignocellulose by the thermotolerant yeast Kluyveromyces marxianus —effects of steam pretreatment conditions, process configurations and substrate loadings. Biochemical Engineering Journal, 79, 94–103.
Kalyani, D., Dhiman, S. S., Kim, H., Jeya, M., Kim, I. W., & Leeab, J. K. (2012). Characterization of a novel laccase from the isolated Coltricia perennis and its application to detoxification of biomass. Process Biochemistry, 47(4), 671–678.
De La Torre, M., Martín-Sampedro, R., Fillat, Ú., Eugenio, M. E., Blánquez, A., Hernández, M., Arias, M. E., & Ibarra, D. (2017). Comparison of the efficiency of bacterial and fungal laccases in delignification and detoxification of steam-pretreated lignocellulosic biomass for bioethanol production. Journal of Industrial Microbiology and Biotechnology, 44(11), 1561–1573.
Saravanakumar, T., Park, H. S., Mo, A. Y., Choi, M. S., Kim, D. H., & Park, S. M. (2016). Detoxification of furanic and phenolic lignocellulose derived inhibitors of yeast using laccase immobilized on bacterial cellulosic nanofibers. Journal of Molecular Catalysis B: Enzymatic, 134, 196–205.
Wei, Z., Zeng, G., Huang, F., Kosa, M., Sun, Q., Meng, X., Huang, D., & Ragauskas, A. J. (2015). Microbial lipid production by oleaginous Rhodococci cultured in lignocellulosic autohydrolysates. Applied Microbiology and Biotechnology, 99(17), 7369–7377.
Huang, C., Wu, H., Li, R. F., & Zong, M. H. (2012). Improving lipid production from bagasse hydrolysate with Trichosporon fermentans by response surface methodology. New Biotechnology, 29(3), 372–378.
Zhang, G. C., French, W. T., Hernandez, R., Alley, E., & Paraschivescu, M. (2011). Effects of furfural and acetic acid on growth and lipid production from glucose and xylose by Rhodotorula glutinis. Biomass and Bioenergy, 35(1), 734–740.
Huang, X., Wang, Y., Liu, W., & Bao, J. (2011). Biological removal of inhibitors leads to the improved lipid production in the lipid fermentation of corn stover hydrolysate by Trichosporon cutaneum. Bioresource Technology, 102, 9705–9709.
Abraham, R. E., Verma, M. L., Barrow, C. J., & Puri, M. (2014). Suitability of magnetic nanoparticle immobilised cellulases in enhancing enzymatic saccharification of pretreated hemp biomass. Biotechnology for Biofuels, 7, 90.
Funding
This work was funded by the Science and Technology Planning Project of Guangdong Province, China (2015A020212033), and University Student Renovation Training Project from South China Normal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yin, L., Chen, J., Wu, W. et al. Immobilization of Laccase on Magnetic Nanoparticles and Application in the Detoxification of Rice Straw Hydrolysate for the Lipid Production of Rhodotorula glutinis. Appl Biochem Biotechnol 193, 998–1010 (2021). https://doi.org/10.1007/s12010-020-03465-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03465-w