Abstract
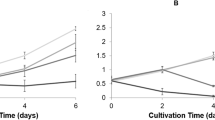
Microorganisms that survive in the high salt environment have been shown to be a potential source for metabolites with pharmaceutical importance. In the present study, we have investigated the effect of 5 and 10% (w/v) NaCl on growth, biochemical changes, and metabolite production in seven moderately halophilic bacteria isolated from the salterns/mangrove area of South India. Metabolite production by Bacillus VITPS3 increased by 3.18-fold in the presence of 10% (w/v) NaCl concentration. Total phenolic and flavonoid content increased in Bacillus VITPS5 (11.3-fold) and Planococcus maritimus VITP21 (5.99-fold) whereas β-carotene content was less at higher NaCl concentrations. VITP21 and VITPS5, in response to NaCl, produced metabolites with higher (6.72- and 4.91-fold) DPPH and ABTS radical scavenging activity. UV/visible spectrophotometry of the extracts confirmed the presence of flavonoids, phenolics, and related compounds. 1H-NMR spectra indicated substantial changes in the metabolite production in response to salt concentration. Principal component analysis (PCA) revealed that VITP21 extracts exhibited the highest antioxidant activity compared with other extracts. The present study presents the first report on the comparative analysis of pigment production by moderate halophilic bacteria, in response to the effect of salt and their relation to radical scavenging property.










Similar content being viewed by others
References
Joghee, N. N., & Jayaraman, G. (2016). Biochemical changes induced by salt stress in halotolerant bacterial isolates are media dependent as well as species-specific. Preparative Biochemistry and Biotechnology, 46(1), 8–14. https://doi.org/10.1080/10826068.2014.970689.
Zhou, A., Lau, R., Baran, R., Ma, J., Von Netzer, F., Shi, W., et al. (2017). Key metabolites and mechanistic changes for salt tolerance in an experimentally evolved sulfate-reducing bacterium, desulfovibrio vulgaris. mBio, 8(6), 1–20. https://doi.org/10.1128/mBio.01780-17.
Abd_Allah, E. F., Alqarawi, A. A., Hashem, A., Radhakrishnan, R., Al-Huqail, A. A., Al-Otibi, F. O. N., & Egamberdieva, D. (2018). Endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. Journal of Plant Interactions, 13(1), 37–44. https://doi.org/10.1080/17429145.2017.1414321.
Modhej, A., & Jamshidi, A. R. (2015). Effect of salt stress on antioxidant activity and seedling growth of three canola ( Brassica napus L .) cultivars. International Journal of Applied Agricultural Research, 31(3), 180–184.
Cabiscol, E., Tamarit, J., & Ros, J. (2000). Oxidative stress in bacteria and protein damage by reactive oxygen species. International Microbiology, 3(1), 3–8. https://doi.org/10.2436/im.v3i1.9235.
Bruno-Bárcena, J. M., Andrea Azcárate-Peril, M., & Hassan, H. M. (2010). Role of antioxidant enzymes in bacterial resistance to organic acids. Applied and Environmental Microbiology, 76(9), 2747–2753. https://doi.org/10.1128/AEM.02718-09.
Lauritano, C., Andersen, J. H., Hansen, E., Albrigtsen, M., Escalera, L., Esposito, F., Helland, K., Hanssen, K. Ø., Romano, G., & Ianora, A. (2016). Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Frontiers in Marine Science, 3(May), 1–12. https://doi.org/10.3389/fmars.2016.00068.
Naziri, D., Hamidi, M., Hassanzadeh, S., Tarhriz, V., Zanjani, B. M., Nazemyieh, H., et al. (2014). Analysis of carotenoid production by Halorubrum sp. TBZ126; an extremely halophilic archeon from Urmia Lake. Advanced Pharmaceutical Bulletin, 4(1), 61–67. https://doi.org/10.5681/apb.2014.010.
Rezaeeyan, Z., Safarpour, A., Amoozegar, M. A., Babavalian, H., Tebyanian, H., & Shakeri, F. (2017). High carotenoid production by a halotolerant bacterium, Kocuria sp. strain QWT-12 and anticancer activity of its carotenoid. EXCLI Journal, 16, 840–851. https://doi.org/10.17179/excli2017-218.
Tam, L. T., Hoang, D. D., Ngoc Mai, D. T., Hoai Thu, N. T., Lan Anh, H. T., & Hong, D. D. (2012). Study on the effect of salt concentration on growth and Astaxanthin accumulation of microalgae Haematococcus pluvialis as the initial basis for two phase culture of astaxanthin production. Tap Chi Sinh Hoc, 34(2), 213–223. https://doi.org/10.15625/0866-7160/v34n2.964.
Valifard, M., Mohsenzadeh, S., Kholdebarin, B., & Rowshan, V. (2014). Effects of salt stress on volatile compounds, total phenolic content and antioxidant activities of Salvia mirzayanii. South African Journal of Botany, 93, 92–97. https://doi.org/10.1016/j.sajb.2014.04.002.
Brunetti, C.,. T. M. (2012). Abiotic stress responses in plants. In Abiotic Stress Responses in. Plants: Metabolism, Productivity and Sustainability. https://doi.org/10.1007/978-1-4614-0634-1.
Yamazaki, G., Nishimura, S., Ishida, A., Kanagasabhapathy, M., Zhou, X., Nagata, S., & Ikeda, T. (2006). Effect of salt stress on pigment production of Serratia rubidaea N-1: A potential indicator strain for screening quorum sensing inhibitors from marine microbes. The Journal of General and Applied Microbiology, 52(2), 113–117. https://doi.org/10.2323/jgam.52.113.
Rad, F. A., Aksoz, N., & Hejazi, M. A. (2011). Effect of salinity on cell growth and β -carotene production in Dunaliella sp . isolates from Urmia Lake in northwest of Iran. African Journal of Biotechnology, 10(12), 2282–2289. https://doi.org/10.5897/AJB10.1934.
Shivanand, P., & Jayaraman, G. (2009). Production of extracellular protease from halotolerant bacterium, Bacillus aquimaris strain VITP4 isolated from Kumta coast. Process Biochemistry, 44, 1088–1094. https://doi.org/10.1016/j.procbio.2009.05.010.
Maier, R. M. (2009). Bacterial growth. Environmental Microbiology, 37–54. https://doi.org/10.1016/B978-0-12-370519-8.00003-1.
Prathiba, S., & Jayaraman, G. (2018). Evaluation of the anti-oxidant property and cytotoxic potential of the metabolites extracted from the bacterial isolates from mangrove Forest and saltern regions of South India. Preparative Biochemistry and Biotechnology, 48(8), 750–758. https://doi.org/10.1080/10826068.2018.1508038.
Blois, M. S. (1958). Antioxidant determinations by the use of a stable free radical [10]. Nature, 181(4617), 1199–1200. https://doi.org/10.1038/1811199a0.
Jayanthi, P., & Lalitha, P. (2011). Reducing power of the solvent extracts of Eichhirnia crassipies (Mart.)Solms. International Journal of Pharmacy and Pharmaceutical Sciences, 3(3), 126–128.
Stanković, N., Mihajilov-Krstev, T., Zlatković, B., Stankov-Jovanović, V., Mitić, V., Jović, J., Čomić, L., Kocić, B., & Bernstein, N. (2016). Antibacterial and antioxidant activity of traditional medicinal plants from the Balkan Peninsula. NJAS - Wageningen Journal of Life Sciences, 78, 21–28. https://doi.org/10.1016/j.njas.2015.12.006.
de Alencar, D. B., de Carvalho, F. C. T., Rebouças, R. H., dos Santos, D. R., dos Santos Pires-Cavalcante, K. M., de Lima, R. L., Baracho, B. M., Bezerra, R. M., Viana, F. A., dos Fernandes Vieira, R. H. S., Sampaio, A. H., de Sousa, O. V., & Saker-Sampaio, S. (2016). Bioactive extracts of red seaweeds Pterocladiella capillacea and Osmundaria obtusiloba (Floridophyceae: Rhodophyta) with antioxidant and bacterial agglutination potential. Asian Pacific Journal of Tropical Medicine, 9(4), 372–379. https://doi.org/10.1016/j.apjtm.2016.03.015.
Annamalai, J., Shanmugam, J., & Nallamuthu, T. (2016). Salt stress enhancing the production of phytochemicals in Chlorella vulgaris and Chlamydomonas reinhardtii. Journal of Algal Biomass Utilization, 7(1), 37–44.
Koek, M. M., Muilwijk, B., Van Der Werf, M. J., & Hankemeier, T. (2006). Microbial metabolomics with gas chromatography/mass spectrometry. Analytical Chemistry, 78(4), 1272–1281. https://doi.org/10.1021/ac051683+.
Baharum, S. N., Bunawan, H., Ghani, A., Aida, W., Mustapha, W., & Noor, N. M. (2010). Analysis of the chemical composition of the essential oil of Polygonum minus Huds. using two-dimensional gas chromatography-time-of-flight mass spectrometry (GC-TOF MS). Molecules, 15(10), 7006–7015. https://doi.org/10.3390/molecules15107006.
Prabhu, M. S., Walawalkar, Y. D., & Furtado, I. (2014). Purification and molecular and biological characterisation of the 1-hydroxyphenazine, produced by an environmental strain of Pseudomonas aeruginosa. World Journal of Microbiology and Biotechnology, 30(12), 3091–3099. https://doi.org/10.1007/s11274-014-1736-7.
Blunder, M., Orthaber, A., Bauer, R., Bucar, F., & Kunert, O. (2017). Efficient identification of flavones, flavanones and their glycosides in routine analysis via off-line combination of sensitive NMR and HPLC experiments. Food Chemistry, 218, 600–609. https://doi.org/10.1016/j.foodchem.2016.09.077.
Sandilyan, S., Thiyagesan, K., Nagarajan, R., & Vencatesan, J. (2010). Salinity rise in Indian mangroves - A looming danger for coastal biodiversity. Current Science, 98(6), 754–756.
Rani, M. H. S., & Kalaiselvam, M. (2018). Diversity of halophilic mycoflora habitat in saltpans of Tuticorin and Marakkanam along southeast coast of India. International Journal of Microbiology and Mycology, 7(1), 1–17.
Pade, N., & Hagemann, M. (2015). Salt acclimation of cyanobacteria and their application in biotechnology. Life, 5(1), 25–49. https://doi.org/10.3390/life5010025.
Abbes, M., Baati, H., Guermazi, S., Messina, C., Santulli, A., Gharsallah, N., & Ammar, E. (2013). Biological properties of carotenoids extracted from Halobacterium halobium isolated from a Tunisian solar saltern. BMC Complementary and Alternative Medicine, 13(1), 13. https://doi.org/10.1186/1472-6882-13-255.
Singh, D. P., Prabha, R., Meena, K. K., Sharma, L., Sharma, A. K., & Singh, D. P. (2014). Induced accumulation of polyphenolics and flavonoids in cyanobacteria under salt stress protects organisms through enhanced antioxidant activity. American Journal of Plant Sciences, 5(5), 726–735. https://doi.org/10.4236/ajps.2014.55087.
Minhas, A. K., Hodgson, P., Barrow, C. J., & Adholeya, A. (2016). A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Frontiers in Microbiology, 7(MAY), 1–19. https://doi.org/10.3389/fmicb.2016.00546.
Guyon, J. B., Vergé, V., Schatt, P., Lozano, J. C., Liennard, M., & Bouget, F. ois Y. (2018). Comparative analysis of culture conditions for the optimization of carotenoid production in several strains of the picoeukaryote ostreococcus. Marine Drugs, 16(3). https://doi.org/10.3390/md16030076.
Ramakrishna, A., & Ravishankar, G. A. (2011). Influence of abiotic stress signals on secondary metabolites in plants. Plant Signaling and Behavior, 6(11), 1720–1731. https://doi.org/10.4161/psb.6.11.17613.
Romano, S., Jackson, S. A., Patry, S., & Dobson, A. D. W. (2018). Extending the “one strain many compounds” (OSMAC) principle to marine microorganisms. Marine Drugs, 16(7), 1–29. https://doi.org/10.3390/md16070244.
Prabhu, S., Pd, R., Young, C. C., Hameed, A., Lin, S. Y., & Ab, A. (2013). Zeaxanthin production by novel marine isolates from coastal sand of India and its antioxidant properties. Applied Biochemistry and Biotechnology, 171(4), 817–831. https://doi.org/10.1007/s12010-013-0397-6.
Babitha, S., Soccol, C. R., & Pandey, A. (2007). Effect of stress on growth, pigment production and morphology of Monascus sp. in solid cultures. Journal of Basic Microbiology, 47(2), 118–126. https://doi.org/10.1002/jobm.200610261.
Gallardo, K., Candia, J. E., Remonsellez, F., Escudero, L. V., & Demergasso, C. S. (2016). The ecological coherence of temperature and salinity tolerance interaction and pigmentation in a non-marine Vibrio isolated from Salar de Atacama. Frontiers in Marine Science, 7(December), 1–10. https://doi.org/10.3389/fmicb.2016.01943.
Benavente-Valdés, J. R., Aguilar, C., Contreras-Esquivel, J. C., Méndez-Zavala, A., & Montañez, J. (2016). Strategies to enhance the production of photosynthetic pigments and lipids in chlorophycae species. Biotechnology Reports, 10, 117–125. https://doi.org/10.1016/j.btre.2016.04.001.
Abubakar, A. L. (2016). Effect of salinity on the growth parameters of halotolerant microalgae, Dunaliella. Nigerian Journal of Basic and Applied Science, 24(2), 85–91.
Brenner, R. R., McMeekin, T. A., Garda, H., Olley, J., & Nichols, D. S. (2002). Effect of temperature and salinity stress on growth and lipid composition of Shewanella gelidimarina. Applied and Environmental Microbiology, 66(6), 2422–2429. https://doi.org/10.1128/aem.66.6.2422-2429.2000.
Langenheder, S., Kisand, V., Wikner, J., & Tranvik, L. J. (2003). Salinity as a structuring factor for the composition and performance of bacterioplankton degrading riverine DOC. FEMS Microbiology Ecology, 45(2), 189–202. https://doi.org/10.1016/S0168-6496(03)00149-1.
Taïbi, K., Taïbi, F., Ait Abderrahim, L., Ennajah, A., Belkhodja, M., & Mulet, J. M. (2016). Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. South African Journal of Botany, 105(July 2016), 306–312. https://doi.org/10.1016/j.sajb.2016.03.011.
Ben Abdallah, S., Aung, B., Amyot, L., Lalin, I., Lachâal, M., Karray-Bouraoui, N., & Hannoufa, A. (2016). Salt stress (NaCl) affects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiologiae Plantarum, 38(3), 1–13. https://doi.org/10.1007/s11738-016-2096-8.
Sévin, D. C., Stählin, J. N., Pollak, G. R., Kuehne, A., & Sauer, U. (2016). Global metabolic responses to salt stress in fifteen species. PLoS One, 11(2), 1–21. https://doi.org/10.1371/journal.pone.0148888.
Azim, N. H., Subki, A., Norhana, Z., Yusof, B., & Sciences, B. (2011). Malaysian Journal of Microbiology. Malaysian journal of microbiology.
Yarsi, G., Sivaci, A., Dasgan, H. Y., Altuntas, O., Binzet, R., & Akhoundnejad, Y. (2017). Effects of salinity stress on chlorophyll and carotenoid contents and stomata size of grafted and ungrafted galia C8 melon cultivar. Pakistan Journal of Botany, 49(2), 421–426.
Babu, M. A., Singh, D., & Gothandam, K. M. (2011). Effect of salt stress on expression of carotenoid pathway genes in tomato effect of salt stress on expression of carotenoid pathway genes in tomato. Journal of Stress Physiology & Biochemistry, 7(3), 87–94.
Abdul Qados, A. M. S. (2011). Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.). Journal of the Saudi Society of Agricultural Sciences, 10(1), 7–15. https://doi.org/10.1016/j.jssas.2010.06.002.
Pawar, R., Mohandass, C., Dastager, S. G., Kolekar, Y. M., & Malwankar, R. (2016). Antioxidative metabolites synthesized by marine pigmented Vibrio sp. and its protection on oxidative deterioration of membrane lipids. Applied Biochemistry and Biotechnology, 179(1), 155–167. https://doi.org/10.1007/s12010-016-1985-z.
Ghasemzadeh, A., Jaafar, H. Z. E., & Rahmat, A. (2010). Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules, 15(6), 4324–4333. https://doi.org/10.3390/molecules15064324.
Borenstein, B., & Bunnell, R. H. (1966). Carotenoids: Properties, occurrence, and utilization in foods. Advances in Food Research, 15(C), 195–276. https://doi.org/10.1016/S0065-2628(08)60081-6.
Esmaeili, A. K., Taha, R. M., Mohajer, S., & Banisalam, B. (2015). Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. (red clover). BioMed Research International, 2015. https://doi.org/10.1155/2015/643285.
Celli, G. B., Pereira-Netto, A. B., & Beta, T. (2011). Comparative analysis of total phenolic content, antioxidant activity, and flavonoids profile of fruits from two varieties of Brazilian cherry (Eugenia uniflora L.) throughout the fruit developmental stages. Food Research International, 44(8), 2442–2451.
Jančič, S., Frisvad, J. C., Kocev, D., Gostinčar, C., Džeroski, S., & Gunde-Cimerman, N. (2016). Production of secondary metabolites in extreme environments: Food- and airborne wallemia spp. produce toxic metabolites at hypersaline conditions. PLoS One, 11(12), 1–20. https://doi.org/10.1371/journal.pone.0169116.
Kol, S., Elena Merlo, M., Scheltema, R. A., De Vries, M., Vonk, R. J., Kikkert, N. A., et al. (2010). Metabolomic characterization of the salt stress response in streptomyces coelicolor. Applied and Environmental Microbiology, 76(8), 2574–2581. https://doi.org/10.1128/AEM.01992-09.
Sen, T., Barrow, C. J., & Deshmukh, S. K. (2019). Microbial pigments in the food industry—Challenges and the way forward. Frontiers in Nutrition, 6(March), 1–14. https://doi.org/10.3389/fnut.2019.00007.
Acknowledgments
The authors wish to thank the management of Vellore Institute of Technology for providing the necessary infrastructure to carry out the study. The authors also wish to acknowledge SIF-Vellore Institute of Technology for GC–MS, and NMR instrumentation facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 488 kb)
Rights and permissions
About this article
Cite this article
Subramanian, P., Gurunathan, J. Differential Production of Pigments by Halophilic Bacteria Under the Effect of Salt and Evaluation of Their Antioxidant Activity. Appl Biochem Biotechnol 190, 391–409 (2020). https://doi.org/10.1007/s12010-019-03107-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-019-03107-w