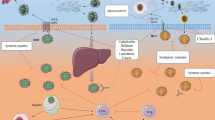
Abstract
Lysozyme is a relatively small enzyme with different biological activities, which is found in tears, saliva, egg white, and human milk. In the study, the anti-HIV-1 activity of lysozymes purified from quail, Meleagris, and hen egg white has been determined. For this end, a time-of-drug-addition assay was performed to identify the target of anti-HIV-1 agents and for determination of probable anti HIV-1 mechanism of the studied lysozyme, the binding affinity of the lysozymes to the human CD4 receptor was studied by molecular docking method. To define structural differences between studied lysozymes, structural motifs of them were predicted by MEME tool. Quail, hen, and Meleagris lysozymes showed potent anti-HIV-1 activity with EC50 of 7.5, 10, and 55 nM, respectively. The time-of-drug-addition study demonstrated that the inhibitory effect of all purified lysozymes is before HIV-1 infection. The frequency and intensity of CD4 expression in PBMCs decreased in the presence of all mentioned lysozymes. Also, the expression level of C-C chemokine receptor type 5 (CCR5) and chemokine receptor type 4 (CXCR4) on CD4+ T cells was not changed in cells treated with these lysozymes. The results of in silico study confirmed that the binding energy of quail lysozyme with CD4 was more than that of other studied lysozymes. The results revealed that these lysozymes restrict HIV-1 attachment to host cell CD4.







Similar content being viewed by others
References
Burley, R. (1989). The avian egg: chemistry and biology.
Feeney, R. E., et al. (1960). The comparative biochemistry of avian egg white proteins. Journal of Biological Chemistry, 235, 2307–2311.
Ting, B., et al. (2010). Fractionation of egg proteins and peptides for nutraceutical applications. Separation, extraction and concentration processes in the food, beverage and nutraceutical industries. p. 595–618.
Abeyrathne, E., Lee, H., & Ahn, D. (2013). Egg white proteins and their potential use in food processing or as nutraceutical and pharmaceutical agents—a review. Poultry Science, 92(12), 3292–3299. https://doi.org/10.3382/ps.2013-03391.
Stevens, L. (1991). Egg white proteins. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 100(1), 1–9. https://doi.org/10.1016/0305-0491(91)90076-P.
Pellegrini, A., et al. (1997). Identification and isolation of a bactericidal domain in chicken egg white lysozyme. Journal of Applied Microbiology, 82(3), 372–378. https://doi.org/10.1046/j.1365-2672.1997.00372.x.
Mine, Y., Ma, F., & Lauriau, S. (2004). Antimicrobial peptides released by enzymatic hydrolysis of hen egg white lysozyme. Journal of Agricultural and Food Chemistry, 52(5), 1088–1094. https://doi.org/10.1021/jf0345752.
Lee-Huang, S., Huang, P. L., Sun, Y., Huang, P. L., Kung, H.f., Blithe, D. L., & Chen, H. C. (1999). Lysozyme and RNases as anti-HIV components in β-core preparations of human chorionic gonadotropin. Proceedings of the National Academy of Sciences, 96(6), 2678–2681. https://doi.org/10.1073/pnas.96.6.2678.
Lee-Huang, S., Huang, P. L., & Huang P. (2010). Anti-HIV and anti-tumor peptides and fragments of lysozyme, Google Patents.
Jin, Q., Chen, H., Wang, X., Zhao, L., Xu, Q., Wang, H., Li, G., Yang, X., Ma, H., Wu, H., & Ji, X. (2015). The effects of the recombinant CCR5 T4 lysozyme fusion protein on HIV-1 infection. PLoS One, 10(7), e0131894. https://doi.org/10.1371/journal.pone.0131894.
Lin, K.-C., Wey, M. T., Kan, L. S., & Shiuan, D. (2009). Characterization of the interactions of lysozyme with DNA by surface plasmon resonance and circular dichroism spectroscopy. Applied Biochemistry and Biotechnology, 158(3), 631–641. https://doi.org/10.1007/s12010-008-8348-3.
Cooke, D. G., & Blackwell, L. F. (2007). Clearing of suspensions of Micrococcus lysodeikticus catalysed by lysozymes from hen, goose, and turkey egg whites, human milk, and phage T4. Assessment of potential as signal generators for homogeneous enzyme immunoassays for urinary steroids. Journal of Immunoassay & Immunochemistry, 28(2), 67–90. https://doi.org/10.1080/15321810701209704.
Ibrahimi, I. M., Prager, E. M., White, T. J., & Wilson, A. C. (1979). Amino acid sequence of California quail lysozyme. Effect of evolutionary substitutions on the antigenic structure of lysozyme. Biochemistry, 18(13), 2736–2744. https://doi.org/10.1021/bi00580a008.
Prager, E. M., Arnheim, N., Mross, G. A., & Wilson, A. C. (1972). Amino acid sequence studies on bobwhite quail egg white lysozyme. Journal of Biological Chemistry, 247(9), 2905–2916.
Liburdi, K., Benucci, I., & Esti, M. (2014). Lysozyme in wine: an overview of current and future applications. Comprehensive Reviews in Food Science and Food Safety, 13(5), 1062–1073. https://doi.org/10.1111/1541-4337.12102.
Lucotte, G., & Kaminski, M. (1978). Increased stability of a hybrid molecule. Journal of Heredity, 69(5), 354–354. https://doi.org/10.1093/oxfordjournals.jhered.a108968.
Thammasirirak, S., Preecharram, S., Ponkham, P., Daduang, S., Araki, T., & Svasti, J. (2007). New variant of quail egg white lysozyme identified by peptide mapping. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 147(2), 314–324. https://doi.org/10.1016/j.cbpb.2007.01.019.
Cegielska-Radziejewska, R., Lesnierowski, G., & Kijowski, J. (2009). Antibacterial activity of hen egg white lysozyme modified by thermochemical technique. European Food Research and Technology, 228(5), 841–845. https://doi.org/10.1007/s00217-008-0997-5.
Cegielska-Radziejewska, R., Lesnierowski, G., Szablewski, T., & Kijowski, J. (2010). Physico-chemical properties and antibacterial activity of modified egg white—lysozyme. European Food Research and Technology, 231(6), 959–964. https://doi.org/10.1007/s00217-010-1347-y.
Cisani, G., Varaldo, P. E., Ingianni, A., Pompei, R., & Satta, G. (1984). Inhibition of herpes simplex virus-induced cytopathic effect by modified hen egg-white lysozymes. Current Microbiology, 10(1), 35–40. https://doi.org/10.1007/BF01576045.
Meng, X.-Y., Zhang, H. X., Mezei, M., & Cui, M. (2011). Molecular docking: a powerful approach for structure-based drug discovery. Current Computer-Aided Drug Design, 7(2), 146–157. https://doi.org/10.2174/157340911795677602.
de Ruyck, J., Brysbaert, G., Blossey, R., & Lensink, M. (2016). Molecular docking as a popular tool in drug design, an in silico travel. Advances and Applications in Bioinformatics and Chemistry: AABC, 9, 1–11. https://doi.org/10.2147/AABC.S105289.
Wang, J., & Hou, T. (2011). Application of molecular dynamics simulations in molecular property prediction. 1. density and heat of vaporization. Journal of Chemical Theory and Computation, 7(7), 2151–2165. https://doi.org/10.1021/ct200142z.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680–685. https://doi.org/10.1038/227680a0.
Shugar, D. (1952). The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochimica et Biophysica Acta, 8(3), 302–309. https://doi.org/10.1016/0006-3002(52)90045-0.
Morgan, D. M. (1998). Tetrazolium (MTT) assay for cellular viability and activity. Polyamine Protocols, p. 179–184.
Tao, J., Hu, Q., Yang, J., Li, R., Li, X., Lu, C., Chen, C., Wang, L., Shattock, R., & Ben, K. (2007). In vitro anti-HIV and-HSV activity and safety of sodium rutin sulfate as a microbicide candidate. Antiviral Research, 75(3), 227–233. https://doi.org/10.1016/j.antiviral.2007.03.008.
Sievers, F., et al. (2011). Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Molecular Systems Biology, 7(1), 539.
Bailey, T. L., et al. (2009). MEME SUITE: tools for motif discovery and searching. Nucleic Acids Research, p. gkp335.
Maehashi, K., Matano, M., Irisawa, T., Uchino, M., Kashiwagi, Y., & Watanabe, T. (2012). Molecular characterization of goose-and chicken-type lysozymes in emu (Dromaius novaehollandiae): evidence for extremely low lysozyme levels in emu egg white. Gene, 492(1), 244–249. https://doi.org/10.1016/j.gene.2011.10.021.
Sakurai, N., Wu, J. H., Sashida, Y., Mimaki, Y., Nikaido, T., Koike, K., Itokawa, H., & Lee, K. H. (2004). Anti-AIDS agents. Part 57: Actein, an anti-HIV principle from the rhizome of Cimicifuga racemosa (black cohosh), and the anti-HIV activity of related saponins. Bioorganic & Medicinal Chemistry Letters, 14(5), 1329–1332. https://doi.org/10.1016/j.bmcl.2003.12.035.
Buonocore, F., Randelli, E., Trisolino, P., Facchiano, A., de Pascale, D., & Scapigliati, G. (2014). Molecular characterization, gene structure and antibacterial activity of a g-type lysozyme from the European sea bass (Dicentrarchus labrax L.) Molecular Immunology, 62(1), 10–18. https://doi.org/10.1016/j.molimm.2014.05.009.
Hashemi, M. M., Aminlari, M., & Moosavinasab, M. (2014). Preparation of and studies on the functional properties and bactericidal activity of the lysozyme–xanthan gum conjugate. LWT-Food Science and Technology, 57(2), 594–602. https://doi.org/10.1016/j.lwt.2014.01.040.
Mateus, L., Costa, L., Silva, Y. J., Pereira, C., & Almeida, A. (2014). Effect of lysozyme addition on the activity of phages against Vibrio parahaemolyticus. Aquaculture, 432, 125–129. https://doi.org/10.1016/j.aquaculture.2014.04.037.
Lambe, T., Leung, J. C. H., Bouriez-Jones, T., Silver, K., Makinen, K., Crockford, T. L., Ferry, H., Forrester, J. V., & Cornall, R. J. (2006). CD4 T cell-dependent autoimmunity against a melanocyte neoantigen induces spontaneous vitiligo and depends upon Fas-Fas ligand interactions. The Journal of Immunology, 177(5), 3055–3062. https://doi.org/10.4049/jimmunol.177.5.3055.
Kaneda, M., Kato, I., Tominaga, N., Titani, K., & Narita, K. (1969). The amino acid sequence of quail lysozyme. The Journal of Biochemistry, 66(5), 747–749.
Lee-Huang, S., Maiorov, V., Huang, P. L., Ng, A., Lee, H. C., Chang, Y. T., Kallenbach, N., Huang, P. L., & Chen, H. C. (2005). Structural and functional modeling of human lysozyme reveals a unique nonapeptide, HL9, with anti-HIV activity. Biochemistry, 44(12), 4648–4655. https://doi.org/10.1021/bi0477081.
Hartono, Y. D., Lee, A. N., Lee-Huang, S., & Zhang, D. (2011). Computational study of bindings of HL9, a nonapeptide fragment of human lysozyme, to HIV-1 fusion protein gp41. Bioorganic & Medicinal Chemistry Letters, 21(6), 1607–1611. https://doi.org/10.1016/j.bmcl.2011.01.121.
Funding
The authors would like to acknowledge the University of Isfahan for the financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Behbahani, M., Nosrati, M. & Mohabatkar, H. Inhibition of Human Immunodeficiency Type 1 Virus (HIV-1) Life Cycle by Different Egg White Lysozymes. Appl Biochem Biotechnol 185, 786–798 (2018). https://doi.org/10.1007/s12010-017-2678-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2678-y