Abstract
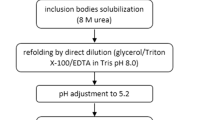
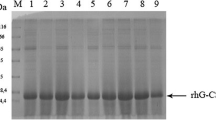
Granulocyte colony-stimulating factor (G-CSF) is a cytokine that has multiple roles in hematopoietic cells such as the regulation of proliferation and differentiation. Here, we describe fed-batch culture, refolding, and purification of rhG-CSF. The suitability of urea or sarcosine for solubilizing inclusion bodies (IBs) was tested. It was observed that urea is more efficient for solubilizing and refolding IBs than sarcosine is. The purity of rhG-CSF and the removal percentage of the rhG-CSF isoforms during purification were increased by pH 5.5 precipitation. The purity and the yield of purified rhG-CSF were 99 % and 0.5 g of protein per liter culture broth, respectively. Our protocols of recombinant protein purification using ion exchange chromatography and semipreparative high performance liquid chromatography of pH-precipitated refolded solution may be informative to the industrial scale production of biopharmaceuticals.







Similar content being viewed by others
References
Barreda, D. R., Hanington, P. C., & Belosevic, M. (2004). Developmental and Comparative Immunology, 28, 509–554.
Nagata, S. (1989). Bioessays, 10, 113–117.
Nicola, N. A., Metcalf, D., Matsumoto, M., & Johnson, G. R. (1983). Journal of Biological Chemistry, 258, 9017–9023.
Williams, G. T., Smith, C. A., Spooncer, E., Dexter, T. M., & Taylor, D. R. (1990). Nature, 343, 76–79.
Rieger, M. A., Hoppe, P. S., Smejkal, B. M., Eitelhuber, A. C., & Schroeder, T. (2009). Science, 325, 217–218.
Hoglund, M. (1998). Medical Oncology, 15, 229–233.
Lu, H. S., Boone, T. C., Souza, L. M., & Lai, P. H. (1989). Archives of Biochemistry and Biophysics, 268, 81–92.
Basu, S., Dunn, A., & Ward, A. (2002). International Journal of Molecular Medicine, 10, 3–10.
Min, C. K., Son, Y. J., Kim, C. K., Park, S. J., & Lee, J. W. (2011). Journal of Biotechnology, 151, 350–356.
Kelker, M. S., Foss, T. R., Peti, W., Teyton, L., Kelly, J. W., Wuthrich, K., & Wilson, I. A. (2004). Journal of Molecular Biology, 342, 1237–1248.
Thomson, C. A., Olson, M., Jackson, L. M., & Schrader, J. W. (2012). PLoS One, 7, e49891.
Schwanke, R. C., Renard, G., Chies, J. M., Campos, M. M., Batista, E. L., Jr., Santos, D. S., & Basso, L. A. (2009). International Journal of Biological Macromolecules, 45, 97–102.
Wang, C., & Geng, X. (2012). Process Biochemistry, 47, 2262–2266.
Rao, D. V., Narasu, M. L., & Rao, A. K. (2008). Biotechnology and Applied Biochemistry, 50, 77–87.
Hubel, K., & Engert, A. (2003). Onkologie, 26, 73–79.
Rubenstein, E. B. (2000). International Journal of Antimicrobial Agents, 16, 117–121.
Wittman, B., Horan, J., & Lyman, G. H. (2006). Cancer Treatment Reviews, 32, 289–303.
Garcia-Carbonero, R., Mayordomo, J. I., Tornamira, M. V., Lopez-Brea, M., Rueda, A., Guillem, V., Arcediano, A., Yubero, A., Ribera, F., Gomez, C., Tres, A., Perez-Gracia, J. L., Lumbreras, C., Hornedo, J., Cortes-Funes, H., & Paz-Ares, L. (2001). Journal of the National Cancer Institute, 93, 31–38.
Walker, J. M. (Ed.). (2002). The protein protocols handbook (2nd ed., pp. 11–14). Totowa: Humana.
Hammerling, U., Kroon, R., & Sjodin, L. (1995). Journal of Pharmaceutical and Biomedical Analysis, 13, 9–20.
Choi, J. H., Jeong, K. J., Kim, S. C., & Lee, S. Y. (2000). Applied Microbiology and Biotechnology, 53, 640–645.
Kim, C. K., Lee, S. M., & Jeong, S. M. (2010). Food Science and Biotechnology, 19, 1627–1633.
Fahnert, B., Lilie, H., & Neubauer, P. (2004). Advances in Biochemical Engineering/Biotechnology, 89, 93–142.
Vanz, A. L., Renard, G., Palma, M. S., Chies, J. M., Dalmora, S. L., Basso, L. A., & Santos, D. S. (2008). Microbial Cell Factories, 7, 13.
Gomes, F. R., Maluenda, A. C., Tapias, J. O., Oliveira, F. L., Sa-Rocha, L. C., Carvalho, E., & Ho, P. L. (2012). World Journal of Microbiology and Biotechnology, 28, 2593–2600.
Wingfield, P., Graber, P., Moonen, P., Craig, S., & Pain, R. H. (1988). European Journal of Biochemistry, 173, 65–72.
Kim, C. K., Lee, C. H., Lee, S. B., & Oh, J. W. (2013). PLoS One, 8, e80109.
Acknowledgments
This work was supported by National Research Foundation of Korea Grant funded by the Korean Government (NRF-2008-313-E00238).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
IEF analyses of rhG-CSF eluted by SP-sepharose chromatography SP-sepharose chromatography was performed to purify rhG-CSF protein. Each fraction obtained was analyzed by Novex® pH 3–10 IEF system. rhG-CSF samples were employed to examine the purity. IEF markers indicate PI. Lane 1, pI marker 4.5–7.4. (PPTX 73.9 kb)
ESM 2
(DOCX 29.5 kb)
Rights and permissions
About this article
Cite this article
Kim, CK., Choi, JH., Lee, SB. et al. Expression and Purification of Recombinant Human Granulocyte Colony-Stimulating Factor in Fed-Batch Culture of Escherichia coli . Appl Biochem Biotechnol 172, 2425–2435 (2014). https://doi.org/10.1007/s12010-013-0708-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0708-y