Abstract
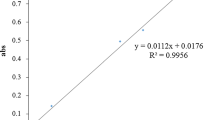
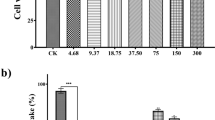
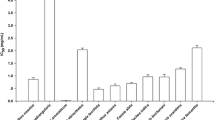
The present study aimed to investigate in vitro biological activities of extract of Eugenia punicifolia leaves (EEP), emphasizing the inhibitory activity of enzymes related to metabolic syndrome and its antioxidant effects. The antioxidant activity was analyzed by free radicals scavengers in vitro assays: DPPH·, ABTS·+, O2 ·−, and NO· and a cell-based assay. EEP were tested in inhibitory colorimetric assays using α-amylase, α-glucosidase, xanthine oxidase, and pancreatic lipase enzymes. The EEP exhibited activity in ABTS·+, DPPH·, and O2 ·− scavenger (IC50 = 10.5 ± 1.2, 28.84 ± 0.54, and 38.12 ± 2.6 μg/mL), respectively. EEP did not show cytotoxic effects, and it showed antioxidant activity in cells in a concentration-dependent manner. EEP exhibited inhibition of α-amylase, α-glucosidase, and xanthine oxidase activities in vitro assays (IC50 = 122.8 ± 6.3; 2.9 ± 0.1; 23.5 ± 2.6), respectively; however, EEP did not inhibit the lipase activity. The findings supported that extract of E. punicifolia leaves is a natural antioxidant and inhibitor of enzymes, such as α-amylase, α-glucosidase, and xanthine oxidase, which can result in a reduction in the carbohydrate absorption rate and decrease of risks factors of cardiovascular disease, thereby providing a novel dietary opportunity for the prevention of metabolic syndrome.




Similar content being viewed by others
Abbreviations
- EEP:
-
Standardized extract of Eugenia punicifolia leaves
- MS:
-
Metabolic syndrome
- DPPH·:
-
2,2-diphenyl-1-picrylhydrazyl
- ABTS+ :
-
2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid
- O2 ·− :
-
Anion superoxide radical
- NO·:
-
Nitric oxide
- HDL:
-
High-density lipoprotein
- LDL:
-
Low-density lipoprotein
- NBT:
-
Nitroblue tetrazolium
- NADH:
-
Nicotinamide-adenine-dinucleotide
- PMS:
-
Phenazine methasulfate
- DCFH-DA:
-
2′,7′-dichlorofluorescin diacetate
- TP:
-
Total polyphenol
- NTF:
-
Non-tannin fraction
- ES:
-
Extractive solution
- TTC:
-
Total tannin content
- DF:
-
Dilution factor
- DMEM:
-
Dulbecco's Modified Eagle Medium
- FBS:
-
Fetal bovine serum
- 4-NPGP:
-
4-nitrophenyl α-d-glucopyranoside
- XO:
-
Xanthine oxidase
- ROS:
-
Reactive oxygen species
References
Ervin B., Ph.D. R.D. (2009) National Health Statistic Report. 5 (13), 1–7.
Holvoet, P. (2008). Verhandelingen-Koninklijke Academie voor Geneeskunde van Belgie., 70(3), 193–219.
Halliwell, B. (1997). Nutrition Reviews, 55(1 Pt 2), S44–S49.
Hansel, B., Giral, P., Nobecourt, E., Chantepie, S., Brucker, E. J., & Kontush, A. (2004). The journal of Clinical Endocrinology & Metabolism, 89(10), 4963–4971.
Cefalu, W. T., Ye, J., Zuberi, A., Ribnicky, D. M., Raskin, I., Liu, Z., et al. (2008). American Journal Clinical Nutrition, 87(2), 481S–487S.
Brunetti, I. L., Vendramini, R. C., Januario, A. H., Franca, S. C., & Pepato, M. T. (2006). Pharmaceutical Journal, 44(1), 35–43.
Voigt, R. (2005). Pharmazeutische Technologie 10. überarb. Aufl., Ullstein Mosby, Berlin
The United States Pharmacopeia. (2000). 25th ed., Mack Printing Company, Easton, PA.
Hartke, K., Mutschler, E. (1987). Deutsches Arzneibuch-9-Kommentar. Ausgabe 1986. Suttgart, Wissenschaftliche.
Mahmoudi, M., Ebrahimzadeh, M., Ansaroudi, F., Nabavi, S. F., & Nabavi, S. M. (2009). Journal of Biotechnology, 8(24), 7170–7175.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Free Radical Biology & Medicine, 26(9/10), 1231–1237.
Öztürk, M., Aydogmus-Öztür, F., Duru, M. E., & Topçu, G. (2007). Food Chemistry, 103(2), 623–630.
Govindarajan, R., Rastogi, S., Vijayakumar, M., Shirwaikar, A., Ajay, K. S. R., Mehrotra, S., et al. (2003). Biological Pharmaceutical Bulletin, 26(10), 1424–1427.
Nakayama, G. R., Caton, M. C., Nova, M. P., & Parandoosh, Z. (1997). Journal of Immunological Methods, 204(2), 205–208.
Wolfe, L. L., & Liu, R. H. (2007). Journal of Agricultural Food Chemistry, 55(22), 8896–8907.
Andrade-Cetto, A., Becerra-Jiménez, J., & Cárdenas-Vázquez, R. (2008). Journal of Ethnopharmacology, 116(1), 27–32.
Subramaniam, R., Asmawi, M. Z., & Sadikun, A. (2008). Acta Biochimica Polonica, 55(2), 391–398.
Bondet, V., Brand-Williams, W., & Berset, C. (1997). Food Science and Technology, 30, 609–615.
Slanc, P., Doljak, B., Kreft, S., Lunder, M., Janes, D., & Strukelj, B. (2009). Phytotherapy Research, 23(6), 874–877.
Espín, J. C., García-Conesa, M. T., & Tomás-Barberán, F. A. (2007). Phytochemistry, 68, 2986–3008.
Cai, Y., Luo, Q., Sun, M., & Corke, H. (2004). Life Sciences, 74(17), 2157–2184.
Apel, K., & Hirt, H. (2004). Annual Review of Plant Biology, 55, 373–399.
Floegel, A., Kim, D., Chung, S., Koo, S., & Chun, O. K. (2011). Journal of food Composition and Analysis, 24, 1043–1048.
Mahmoudi, M., Ebrahimzadeh, M., Ansaroudi, F., Nabavi, S. F., & Nabavi, S. M. (2009). Journal of Biotechnology, 8(24), 7170–7175.
Ilhami, G., Bursal, E., Sehitoglu, M. H., Bilsel, M., & Goren, A. C. (2010). Food and Chemical Toxicology, 48(8–9), 2227–2238.
Formica, J. V., & Regelson, W. (1995). Food and Chemical Toxicology, 33(12), 1061–1080.
Rice-evans, C. A., & Miller, N. J. (1996). Biochemical Society Transactions, 24(3), 790–795.
Hung, H. C., Joshipura, K. J., Jiang, R., Hu, F. B., Hunter, D., Smith-Warner, S. A., et al. (2004). Journal of the National Cancer Institute, 96(21), 1577–1584.
Shim, Y. J., Doo, H. K., Ahn, S. Y., Kim, Y. S., Seong, J. K., Park, I. S., et al. (2003). Journal of Ethnopharmacology, 85, 283–287.
Van de Laar, F., Lucassen, P. L., Akkermans, R. P., Van de Lisdonk, E. H., Rutten, G. E., & Van Weel, C. (2005). Diabetes Care, 28, 154–163.
Kim, S. H., Sung-Hoon, J. O., Young-In, K., & Jae-Kwan, H. (2011). International Journal of Molecular Science, 12(6), 3757–3769.
Ahmed, F., Chandra, J. N. N. S., & Timmaiah, N. V. (2009). Pharmacognos, 1(4), 317–321.
Adyanthaya, I., Kwon, Y. I., Apostolidis, E., & Shetty, K. (2010). Journal of food Biochemistry, 34(1), 31–49.
Lin, C. C., Huang, P. C., & Lin, J. M. (2000). The American Journal Chinese Medicine, 28(1), 87–96.
Heber, D., Seeram, N., Wyatt, H., Henning, S. M., Zhang, Y., Ogden, L. G., et al. (2007). Journal of Agricultural and Food Chemistry, 55(24), 0050–10054.
Zajácz, A., Gyémánt, G., Vittori, N., & Kandra, L. (2007). Carbohydrate Research, 342, 717–723.
Consolini, A. E., & Sarubio, M. G. (2002). Journal of Ethnopharmacology, 81(1), 57–63.
Acknowledgments
The authors are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM) for the financial support of this research. ESL is member of the INCT of Processes Redox in Biomedicina-Redoxoma (MCT/CNPq). APAB received a grant from DCR/CNPq/FAPEAM. Thanks to Célio Maia Chaves from EMBRAPA for the Eugenia punicifolia plant material donation and to Jim Hesson of AcademicEnglishSolutions.com for proofreading the English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galeno, D.M.L., Carvalho, R.P., de Araújo Boleti, A.P. et al. Extract from Eugenia punicifolia is an Antioxidant and Inhibits Enzymes Related to Metabolic Syndrome. Appl Biochem Biotechnol 172, 311–324 (2014). https://doi.org/10.1007/s12010-013-0520-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-013-0520-8