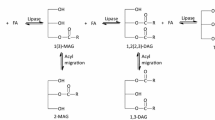
Abstract
This work reports the experimental data and kinetic modeling of diacylglycerol (DAG) production from palm oil using a commercial immobilized lipase (Lipozyme RM IM) in a solvent-free medium. The experiments were performed in batch mode, at 55 °C and 400 rpm, and the effects of enzyme concentration (0.68–2.04 wt% related to the mass of substrates), initial water concentration (5–15 wt% related to the mass of oil), and reaction time were evaluated. A novel kinetic model is presented based on the ordered-sequential bi–bi mechanism considering hydrolysis and esterification steps, in which a correlation between water-in-oil solubility and surfactant molecules concentration in the oil allowed the model to describe the induction period in the beginning of the hydrolysis reaction. Moreover, mass transfer limitations related to the enzyme concentration in the system were also taken into account. The proposed model presented a very satisfactory agreement with the experimental data, thus allowing a better understanding of the reaction kinetics. The best conditions obtained for the product (partially hydrolyzed palm oil) in terms of DAG yield (35.91 wt%) were 2.87 wt% enzyme/substrate, 2.10 wt% water/oil, and 72 h of reaction.







Similar content being viewed by others
Abbreviations
- a r :
-
Relative activity
- DAG:
-
Diacylglycerol
- E:
-
Free enzyme
- EMM:
-
Enzyme molar mass (grams per mole)
- fc:
-
Correction factor
- FFA:
-
Free fatty acid
- G:
-
Glycerol
- H2O:
-
Water in the oil phase
- H2Oins :
-
Water not solubilized in the oil
- H2Ot :
-
Total water in the system
- k l :
-
Mass transfer limitation constant (grams substrate per gram enzyme)
- k r (r = 1,…,22):
-
Rate constants
- K r (r = 1,…,7):
-
Apparent rate constants (g substrate2/mmol2)
- MAG:
-
Monoacylglycerol
- NMR:
-
Nuclear magnetic resonance
- NOBS:
-
Number of observations
- M FFA :
-
Molar mass of the free fatty acid of palm oil (grams per mole)
- M DAG :
-
Molar mass of the diacylglycerol of palm oil (grams per mole)
- M MAG :
-
Molar mass of the monoacylglycerol of palm oil (grams per mole)
- M TAG :
-
Molar mass of the triacylglycerol of palm oil (grams per mole)
- Q r (r = 1,…,6):
-
Apparent rate constants (g substrate2/(mol enzyme h mmol))
- rmsd:
-
Root–mean–square deviation
- TAG:
-
Triacylglycerol
- V r (r = 1,…,6):
-
Apparent rate constants (g substrate2/(g enzyme h mmol))
- \( \left[ {x_{\text{surf}}^{\text{oil}}} \right] \) :
-
Molar fraction of surfactants in the oil
- \( \left[ {w_{{io}}^{{\exp }}} \right] \) :
-
Experimental mass fraction of pseudo-component i in observation o
- \( \left[ {w_{{io}}^{\text{calc}}} \right] \) :
-
Calculated mass fraction of pseudo-component i in observation o
- [DAG]:
-
Diacylglycerol concentration (millimoles per gram substrate)
- [DAG × E × FFA]:
-
Diacylglycerol × enzyme × free fatty acid complex concentration (millimoles per gram substrate)
- [DAG × E × H2O]:
-
Diacylglycerol × enzyme × water complex concentration (millimoles per gram substrate)
- [E]:
-
Free enzyme concentration (millimoles per gram substrate)
- [ET]:
-
Total enzyme concentration (millimoles per gram substrate)
- [ET]:
-
Total enzyme concentration (grams enzyme per gram substrate)
- [ETa]:
-
Total enzyme concentration available to substrate (grams enzyme per gram substrate)
- [FFA]:
-
Free fatty acids concentration (millimoles per gram substrate)
- [H2O]:
-
Water concentration in the oil phase (millimoles per gram substrate)
- [H2O]0 :
-
Initial water concentration in the oil phase (millimoles per gram substrate)
- [H2Oins]:
-
Concentration of water not solubilized in the oil phase (millimoles per gram substrate)
- [H2Oins]0 :
-
Initial concentration of water not solubilized in the oil phase (millimoles per gram substrate)
- [H2Ot]:
-
Total water concentration in the system (millimoles per gram substrate)
- [H2Ot]0 :
-
Total initial water concentration in the system (millimoles per gram substrate)
- [G]:
-
Glycerol concentration (millimoles per gram substrate)
- [G × E × FFA]:
-
Glycerol × enzyme × free fatty acid complex concentration (millimoles per gram substrate)
- [MAG]:
-
Monoacylglycerol concentration (millimoles per gram substrate)
- [MAG × E × FFA]:
-
Monoacylglycerol × enzyme × free fatty acid complex concentration (millimoles per gram substrate)
- [MAG × E × H2O]:
-
Monoacylglycerol × enzyme × water complex concentration (millimoles per gram substrate)
- [TAG]:
-
Triacylglycerol concentration (millimoles per gram substrate)
- [TAG × E × H2O]:
-
Triacylglycerol × enzyme × water complex concentration (millimoles per gram substrate)
References
Cheong, L. Z., Tan, C. P., Long, K., Yussof, M. S. A., Arifin, N., Lo, S. K., & Lai, O. M. (2007). Production of a diacylglycerol-enriched palm olein using lipase-catalyzed partial hydrolysis: Optimization using response surface methodology. Food Chemistry, 105, 1614–1622.
Yang, T., Zhang, H., Mu, H., Sinclair, A. J., & Xu, X. (2004). Diacylglycerols from butterfat: Production by glycerolysis and short path distillation and analysis of physical properties. Journal of the American Oil Chemists' Society, 81, 979–987.
Kawashima, H., Takase, H., Yasunaga, K., Wakaki, Y., Katsuragi, Y., Mori, K., Yamaguchi, T., Hase, T., Matsuo, N., Yasukawa, T., Tokimitsu, I., & Koyama, W. (2008). One-year ad libitum consumption of diacylglycerol oil as part of a regular diet results in modest weight loss in comparison with consumption of a triacylglycerol control oil in overweight japanese subjects. Journal of the American Dietetic Association, 108, 57–66.
Taguchi, H., Watanabe, H., Onizawa, K., Nagao, T., Gotoh, N., Yasukawa, T., Tsushima, R., Shimasaki, H., & Itakura, H. (2000). Double-blind controlled study on the effects of dietary diacylglycerol on postprandial serum and chylomicron triacylglycerol responses in healthy humans. Journal of the American College of Nutrition, 19, 789–796.
Flickinger, B. D., & Matsuo, N. (2003). Nutritional characteristics of DAG oil. Lipids, 38, 129–132.
Ferreira-Dias, S., Correia, A. C., Baptista, F. O., & Fonseca, M. M. R. (2001). Contribution of response surface design to the development of glycerolysis systems catalyzed by commercial immobilized lipases. Journal of Molecular Catalysis B: Enzymatic, 11, 699–711.
Sonntag, N. O. V. (1982). Glycerolysis of fats and methyl esters-status, reviews and critique. Journal of the American Oil Chemists' Society, 59, 795–802.
Tuter, M., & Aksoy, H. A. (2000). Solvent-free glycerolysis of palm and palm kernel oils catalyzed by commercial 1,3-specific lipase from Humicola lanuginosa and composition of glycerolysis products. Biotechnology Letters, 22, 31–34.
Freitas, L., Bueno, T., Perez, V. H., & Castro, H. F. (2008). Monoglicerídeos: Produção por via enzimática e algumas aplicações. Quim Nova, 31, 1514–1521.
Berger, M., Laumen, K., & Schneider, M. P. (1992). Enzymatic esterification of glycerol I. Lipase catalyzed synthesis of regioisomerically pure 1,3-sn-diacyglycerols. Journal of the American Oil Chemists' Society, 69, 955–960.
Lo, S. K., Baharin, B. S., Tan, C. P., & Lai, O. M. (2004). Lipasecatalyzed production and chemical composition of diacylglycerols from soybean oil deodorizer distillate. European Journal of Lipid Science and Technology, 106, 218–224.
Watanabe, T., Shimizu, M., Sugiura, M., Sato, M., Kohori, J., Yamada, N., & Nakanishi, K. (2003). Optimization of reaction conditions for the production of DAG using immobilized 1,3-regiospecific lipase Lipozyme RM IM. Journal of the American Oil Chemists' Society, 80, 1201–1207.
Kristensen, J. B., Xu, X., & Mu, H. (2005). Diacylglycerol synthesis by enzymatic glycerolysis: Screening of commercially available lipase. Journal of the American Oil Chemists' Society, 82, 329–334.
Zhao, Y., Liu, J., Deng, L., Wang, F., & Tan, T. (2011). Optimization of Candida sp. 99–125 lipase catalyzed esterification for synthesis of monoglyceride and diglyceride in solvent-free system. Journal of Molecular Catalysis B: Enzymatic, 72, 157–162.
Yamane, T., Kang, S. T., Kawahara, K., & Koizumi, Y. (1994). High yield diacyglycerol formation by solid phase enzymatic glycerolysis of hydrogenated beef tallow. Journal of the American Oil Chemists' Society, 71, 339–342.
Voll, F., Kruger, R. L., Castilhos, F., Cardozo-Filho, L., Cabral, V., Ninow, J., & Corazza, M. L. (2011). Kinetic modeling of lipase-catalyzed glycerolysis of olive oil. Biochemical Engineering Journal, 56, 107–115.
Rosu, R., Iwasaki, Y., Shimidzu, N., Doisaki, N., & Yamane, T. (1998). Enzymatic synthesis of glycerides from DHA-enriched PUFA ethyl ester by glycerolysis under vacuum. Journal of Molecular Catalysis B: Enzymatic, 4, 191–198.
Plou, F. J., Barandiaran, M., Calvo, M. V., Mallesteros, A., & Pastor, E. (1996). High yield production of mono and diolelyglycerol by lipase catalyzed hydrolysis of triolein. Enzyme and Microbial Technology, 18, 66–71.
Matos, L. M. C., Leal, I. C. R., & Souza, R. O. M. A. (2011). Diacylglycerol synthesis by lipase-catalyzed partial hydrolysis of palm oil under microwave irradiation and continuous flow conditions. Journal of Molecular Catalysis B: Enzymatic, 72, 36–39.
Pawongrat, R., Xu, X., & H-Kittikun, A. (2007). Synthesis of monoacylglycerol rich in polyunsaturated fatty acids from tuna oil with immobilized lipase AK. Food Chemistry, 104, 251–258.
Kaewthong, W., & H-Kittikun, A. (2004). Glycerolysis of palm olein by immobilized lipase PS in organic solvents. Enzyme and Microbial Technology, 35, 218–222.
Dossat, V., Combes, D., & Marty, A. (2002). Lipase-catalysed transesterification of high oleic sunflower oil. Enzyme and Microbial Technology, 30, 90–94.
Chatterjee, T., & Bhattacharyya, D. K. (1998). Synthesis of terpene esters by an immobilized lipase in a solvent-free system. Biotechnology Letters, 20, 865–868.
Ghamghi, H., Karra-Chaabouni, M., & Gargori, Y. (2004). 1-Butyl oleate synthesis by immobilized lipase from Rhizopus oryzae: A comparative study between nhexane and solventfree system. Enzyme and Microbial Technology, 35, 355–363.
Moquin, P. H. L., & Temelli, F. (2008). Kinetic modeling of hydrolysis of canola oil in supercritical media. Journal of Supercritical Fluids, 45, 94–101.
Lam, M. K., Tan, K. T., Lee, K. T., & Mohamed, A. R. (2009). Malaysian palm oil: Surviving the food versus fuel dispute for a sustainable future. Renewable and Sustainable Energy Reviews, 13, 1456–1464.
Edem, D. O. (2002). Palm oil: Biochemical, physiological, nutritional, hematological, and toxicological aspects: A review. Plant Foods for Human Nutrition, 57, 319–341.
O’Brien, R. D. (2003). Fats and oils: Formulating and processing for applications (2nd ed.). Boca Raton: CRC.
Valério, A., Krüger, R. L., Ninow, J., Corazza, F. C., Oliveira, D., Oliveria, J. V., & Corazza, M. L. (2009). Kinetics of solvent-free lipase-catalyzed glycerolysis of olive oil in surfactant system. Journal of Agricultural and Food Chemistry, 57, 8350–8356.
Cheirsilp, B., Kaewthong, W., & H-Kittikun, A. (2007). Kinetic study of glycerolysis of palm olein for monoacylglycerol production by immobilized lipase. Biochemical Engineering Journal, 35, 71–80.
Rukunudin, I. H., White, P. J., Bern, C. J., & Bailey, T. B. (1998). A modified method for determining free fatty acids from small soybean oil sample sizes. Journal of the American Oil Chemists' Society, 75, 563–568.
Ng, S. (2000). Quantitative analysis of partial acylglycerols and free fatty acids in palm oil by 13C nuclear magnetic resonance spectroscopy. Journal of the American Oil Chemists' Society, 77, 749–755.
Gunstone, F. D. (1991). 13C-NMR studies of mono-, di- and triacylglycerols leading to qualitative and semiquantitative information about mixtures of these glycerol esters. Chemistry and Physics of Lipids, 58, 219–224.
Chen, J. P., & Wang, H. Y. (1998). Improved properties of bilirubin oxidase by entrapment in alginate–silicate sol–gel matrix. Biotechnology Techniques, 12, 851–853.
Oliveira, M. A. (2007). Dissertação (Mestrado em Química)—Setor de Ciências Exatas. Curitiba (In Portuguese): Universidade Federal do Paraná.
Romero, M. D., Calvo, L., Alba, C., & Daneshfar, A. (2007). A kinetic study of isoamyl acetate synthesis by immobilized lipase-catalyzed acetylation in n-hexane. Journal of Biotechnology, 127, 269–277.
Satyarthi, J. K., Srinivas, D., & Ratnasamy, P. (2011). Hydrolysis of vegetable oils and fats to fatty acids over solid acid catalysts. Applied Catalysis A, 391, 427–435.
Valério, A., Fiametti, K. G., Rovani, S., Franceschi, E., Corazza, M. L., Treichel, H., Oliveira, D., & Oliveira, J. V. (2009). Enzymatic production of mono- and diglycerides in compressed n-butane and AOT surfactant. Journal of Supercritical Fluids, 49, 216–220.
Noor, I. M., Hasan, M., & Ramachandran, K. B. (2003). Effect of operating variables on the hydrolysis rate of palm oil by lipase. Process Biochemistry, 39, 13–20.
Acknowledgments
The authors are grateful to CNPq and CAPES/NANOBIOTEC for the financial support and scholarships.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Voll, F.A.P., Zanette, A.F., Cabral, V.F. et al. Kinetic Modeling of Solvent-Free Lipase-Catalyzed Partial Hydrolysis of Palm Oil. Appl Biochem Biotechnol 168, 1121–1142 (2012). https://doi.org/10.1007/s12010-012-9846-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9846-x