Abstract
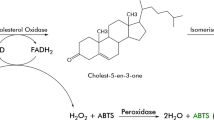
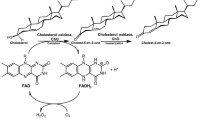
An extracellular cholesterol oxidase (cho) enzyme was isolated from the Streptomyces parvus, a new source and purified 18-fold by ion exchange and gel filtration chromatography. Specific activity of the purified enzyme was found to be 20 U/mg with a 55 kDa molecular mass. The enzyme was stable at pH 7.2 and 50 °C. The enzyme activity was inhibited in the presence of Pb2+, Ag2+, Hg2+, and Zn2+ and enhanced in the presence of Mn2+. The enzyme activity was inhibited by the thiol-reducing reagents (DTT, β-mercaptoethanol), suggesting that disulfide linkage is essential for the enzyme activity. The enzyme activity was found to be maximum in the presence of Triton X-100 and X-114 detergents whereas sodium dodecyl sulfate fully inactivated the enzyme. The enzyme showed moderate stability towards all organic solvents except acetone, benzene, chloroform and the activity increased in the presence of isopropanol and ethanol. The K m value for the oxidation of cholesterol by this enzyme was 0.02 mM.









Similar content being viewed by others
References
Smith, A. J., & Brooks, C. J. W. (1974). Journal of Chromatography, 101, 373–378.
Hino, K. (1996). Clinical Chemistry, 42, 296–299.
Kauntiz, H. (1978). Lipids, 13, 373–374.
Watanabe, K., Shimizu, H., Aihara, H., Nakamura, R., & Suzuki, K. (1986). Komagata, K. Applied Microbiology, 32, 137–147.
Purcell, J. P. (1993). Biochemical Biophysical Research Communication, 196, 1406–1413.
Mendes, M. V. (2007). Chemistry and Biology, 14, 279–290.
Kamei, T., Takiguchi, Y., Suzuki, H., Matsuzaki, M., & Nakamura, S. (1978). Chemical and Pharmaceutical Bulletin, 26, 2799–2804.
Lolekha, P. H., & Jantaveesirirat, Y. (1992). Journal of Clinical Laboratory Analysis, 6, 405–409.
Nishiya, Y. (1997). Protein Engineering, 10, 231–235.
Shirling, E. B., & Gottlieb, D. (1966). Interational Journal of Systematic Bacteriology, 16, 313–340.
Holt, J. G., Krieg, N. R., Sneath, P. H. A., Staley, J. T., & Williams, S. T. (1994). Bergey’s manual of determinative bacteriology (9th ed.). Baltimore: William and Wilkins.
Castillo, U. F., & Strobel, G. A. (2002). Microbiology, 148, 2675–2685.
Praveen, V., & Tripathi, C. K. M. (2009). Letters in Applied Microbiology, 49, 450–455.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Journal of Molecular Biology, 215, 403–410.
Saitou, N., & Nei, M. (1987). Molecular Biology and Evolution, 4, 406–425.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Journal of Biological Chemistry, 193, 265–275.
Fujishiro, K. (2002). FEMS Microbiology Letters, 215, 243–248.
Laemmli, U. K. (1970). Nature, 224, 680–685.
Yazdi, M. T., Zahraei, M., Aghaepour, K., & Kamranpour, N. (2001). Enzyme and Microbial Technology, 28, 410–414.
Uwajima, T., Yagi, H., & Terada, O. (1974). Agricultural and Biological Chemistry, 83, 1149–1156.
Wang, C., Cao, Y., Sun, B., Ji, B., Nout, R., Wang, Ji, et al. (2008). World Journal of Microbiology and Biotechnology, 24, 2149–2157.
Lee, S., Rhee, H., Tae, W., Shin, J., & Park, B. (1989). Applied Microbiology and Biotechnology, 31, 542–546.
Isobe, K., Shoji, K., Nakanishi, Y., Yokoe, M., & Wakao, N. (2003). Journal of Bioscience and Bioengineering, 95, 257–263.
Guo, L. W., Wilson, W. K., Pang, J., & Shackleton, C. H. (2003). Steroids, 68, 31–42.
Lineweaver, H., & Burk, D. (1934). Journal of American Chemical Society, 56, 658–666.
Srisawasdi, P., Srisawasdi, P., Jearanaikoon, P., Kroll, M. H., & Lolekha, P. H. (2005). Journal of Clinical Laboratory Analysis, 19, 247–252.
Acknowledgments
This study was financially supported by the Council of Scientific and Industrial Research, India.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Praveen, V., Srivastava, A. & Tripathi, C.K.M. Purification and Characterization of the Enzyme Cholesterol Oxidase from a New Isolate of Streptomyces sp.. Appl Biochem Biotechnol 165, 1414–1426 (2011). https://doi.org/10.1007/s12010-011-9360-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9360-6