Abstract
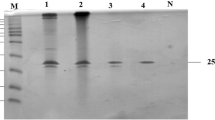
Two xylanases from the crude culture filtrate of Penicillium sclerotiorum were purified to homogeneity by a rapid and efficient procedure, using ion-exchange and molecular exclusion chromatography. Molecular masses estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis were 23.9 and 33.1 kDa for xylanase I and II, respectively. The native enzymes’ molecular masses of 23.8 and 30.8 kDa were estimated for xylanase I and II, respectively, by molecular exclusion chromatography. Both enzymes are glycoproteins with optimum temperature and pH of 50 °C and pH 2.5 for xylanase I and 55 °C and pH 4.5 for xylanase II. The reducing agents β-mercaptoethanol and dithio-treitol enhanced xylanase activities, while the ions Hg2+ and Cu2+ as well the detergent SDS were strong inhibitors of both enzymes, but xylanase II was stimulated when incubated with Mn2+. The K m value of xylanase I for birchwood xylan and for oat spelt xylan were 6.5 and 2.6 mg mL−1, respectively, whereas the K m values of xylanase II for these substrates were 26.61 and 23.45 mg mL−1. The hydrolysis of oat spelt xylan by xylanase I released xylobiose and larger xylooligosaccharides while xylooligosaccharides with a decreasing polymerization degree up to xylotriose were observed by the action of xylanase II. The present study is among the first works to examine and describe an extracellular, highly acidophilic xylanase, with an unusual optimum pH at 2.5. Previously, only one work described a xylanase with optimum pH 2.0. This novel xylanase showed interesting characteristics for biotechnological process such as feed and food industries.








Similar content being viewed by others
References
Biely, P. (1985). Trends in Biotechnology, 3, 286–290.
Kulkarni, N., Shendye, A., & Rao, M. (1999). FEMS Microbiology Reviews, 23, 411–456.
Collins, T., Gerday, C., & Feller, G. (2005). FEMS Microbiology Reviews, 29, 3–23.
Beg, Q. K., Kapoor, M., Mahajan, L., & Hoondal, G. S. (2001). Applied Microbiology Biotechnology, 56, 326–338.
Wong, K. K. Y., Tan, L. U. L., & Saddler, J. N. (1988). Microbiological Reviews, 52, 305–317.
Sunna, A., & Antranikian, G. (1997). Critical Reviews in Biotechnology, 17, 39–67.
Chandrakant, P., & Bisaria, B. S. (1998). Critical Reviews in Biotechnology, 18, 295–331.
Matt, J., Roza, M., Verbakel, J., Stam, H., da Silra, M. J. S., Egmond, M. R., et al. (1992). In J. Visser, G. Beldman, M. A. Kursters-van Someren & A. G. J. Voragen (Eds.), Xylan and xylanases (pp. 349–360). Amsterdam: Elsevier.
Polizeli, M. L. T. M., Rizzati, A. C. S., Monti, R., Terenzi, H. F., Jorge, J. A., & Amorin, D. S. (2005). Applied Microbiology Biotechnology, 67, 577–91.
Krisana, A., Rutchadaporn, S., Jarupan, G., Lily, E., Sutipa, T., & Kanyawim, K. (2005). Journal of Biochemistry and Molecular Biology, 38, 17–23.
Moss, M. O. (1987). In J. F. Peberdy (Ed.), Penicillium and Acremonium (pp. 37–71). New York: Plenum.
Chávez, R., Bull, P., & Eyzaguirre, J. (2006). Journal of Biotechnology, 123, 413–433.
Knob, A., & Carmona, E. C. (2008). World Applied Sciences Journal, 4(227), 283.
Vogel, H. J. (1956). Microbial Genetics Bulletin, 13, 42–43.
Miller, G. L. (1959). Analytical Chemistry, 31, 426–429.
Lowry, O. H., Rosebrough, N. F., Farr, A. L., & Randall, R. J. (1951). Journal of Biological Chemistry, 193, 265–275.
Laemmli, U. K. (1970). Nature, 227, 680–685.
Dubois, M., Gilles, K. A., Hamilton, J. K., Ribers, P. A., & Smith, F. (1956). Analytical Chemistry, 58, 350–356.
Fontana, J. D., Geabrara, M., Blumel, M., Schneider, H., Mackenzie, C. R., & Johnson, H. K. (1988). Methods in Enzymology, 160, 560–571.
Törrönem, A., & Rouvine, J. (1997). Journal of Biotechnology, 57, 137–149.
Segura, B. G., & Fevre, M. (1993). Applied and Environmental Microbiology, 59, 3654–3660.
Nair, S. G., Sindhu, R., & Shashidhar, S. (2008). Applied Biochemistry Biotechnology, 149, 229–243.
Fialho, M. B., & Carmona, E. C. (2004). Folia Microbiologica, 49, 13–18.
Carmona, E. C., Brochetto-Braga, M. R., Pizzirani-Kleiner, A. A., & Jorge, J. A. (1998). FEMS Microbiology Letters, 166, 311–315.
Carmona, E. C., Fialho, M. B., Buchgnani, E. B., Coelho, G. D. C., Brochetto-Braga, M. R., & Jorge, J. A. (2005). Process Biochemistry, 40, 359–364.
Flannigan, B., & Sellars, P. N. (1977). Transaction of the British Mycological Society, 69, 316–317.
Li, L., Hongmei, T., Cheng, Y., Jiang, Z., & Yang, S. (2006). Enzyme and Microbial Technology, 38, 780–787.
Krishnamurthy, S., & Vithayathil, P. J. (1989). Journal of Fermentation and Bioengineering, 67, 77–82.
Amoresano, A., Andolfo, A., Corsaro, M. M., Zocchi, I., Petrescu, I., Gerday, C., et al. (2000). Glycobiology, 10, 451–458.
Romanowska, I., Polak, J., & Bielecki, S. (2006). Applied Microbiology Biotechnology, 69, 665–671.
Sadrim, V. C., Rizzatti, A. C. S., Terenzi, H. F., Jorge, J. A., Milagres, A. M. F., & Polizeli, M. L. T. M. (2005). Process Biochemistry, 40, 1823–1828.
Saha, B. C. (2001). Applied Microbiology Biotechnology, 56, 762–766.
Dutta, T., Sengupta, R., Sahoo, R., Ray, S. S., Bhattacharjee, A., & Ghosh, S. (2007). Letters in Applied Microbiology, 44, 206–211.
Kimura, T., Ito, J., Kawano, A., Makino, T., Kondo, H., Karita, S., et al. (2000). Bioscience Biotechnology and Biochemistry, 64, 1230–1237.
Lee, J.-W., Park, J.-Y., Kwon, M., & Choi, I.-G. (2009). Journal of Bioscience and Bioengineering, 107, 33–37.
Madlala, A. M., Bissoon, S., Singh, S., & Christov, L. (2001). Biotechnology Letters, 23, 345–351.
Hakulinen, N., Turunen, O., Jamis, J., Leisola, M., & Rouvinen, J. (2003). European Journal of Biochemistry, 270, 1399–1412.
Fengxia, L., Mei, L., Zhaoxin, L., Xiaomei, B., Zhao, H., & Wang, Y. (2008). Bioresource Technology, 99, 5983–5941.
Bastawde, K. B. (1992). World Journal of Microbiology and Biotechnology, 8, 353–368.
Kang, M. K., Maeng, P. J., & Rhee, Y. H. (1996). Applied and Environmental Microbiology, 62, 3480–3482.
Haas, H., Herfurth, E., Stoffler, G., & Rendl, B. (1992). Acta Biochimica et Biophysica Sinica, 117, 279–286.
Li, K., Azadi, P., Collins, R., Tolan, J., Kim, J. S., & Eriksoon, K. E. L. (2000). Enzyme and Microbial Technology, 27, 89–94.
Jorge, I., Rosa, O., Navas-Cortés, J. A., Jiménez-Días, R. M., & Tena, M. (2005). Antonie van Leeuwenhoek, 88, 49–59.
Cardoso, O. A. V., & Filho, E. X. F. (2003). FEMS Microbiology Letters, 223, 309–314.
Bedford, M. R., & Classen, H. L. (1992). In J. Visser, G. Beldman, M. A. Kursters-van Someren & A. G. J. Voragen (Eds.), Xylan and xylanases (pp. 361–370). Amsterdam: Elsevier.
Graminha, E. B. N., Gonçalves, A. Z. L., Pirota, R. D. P. B., Balsalobre, M. A. A., Da Silva, R., & Gomes, E. (2008). Animal Feed Science and Technology, 144, 1–22.
Beauchemim, K. A., Colombatto, D., Morgavi, D. P., & Yang, W. Z. (2003). Journal of Animal Science, 81, E37–E47.
Eijsink, V. G. H., Gaseidnes, S., Borchert, T. V., & van den Burg, B. (2005). Biomolecular Engineering, 22, 21–30.
Biely, P. (1985). Trends in Biotechnology, 3, 286–290.
Colagrande, O., Silva, A., & Fumi, M. D. (1994). Biotechnology Progress, 10, 2–18.
Moure, A., Gullon, P., Dominguez, H., & Parajó, J. C. (2006). Process Biochemistry, 41, 1913–1923.
Chen, C. S., Chen, J. L., & Lin, T. Y. (1997). Enzyme and Microbial Technology, 21, 91–96.
Eneyskaya, E. V., Brumer, L. V., Backinowsky, D. R., Ivanen, A. A., Kulminskaya, K. A., Shabalin, K. A., et al. (2003). Carbohydrate Research, 338, 213–325.
Jiang, Z., Zhu, Y., Li, L., Yu, X., Kusakabe, I., Kitaoka, M., et al. (2004). Journal of Biotechnology, 114, 125–134.
Kurakate, M., Fujii, T., Yata, M., Okazaki, T., & Komaki, T. (2005). Biochimica et Biophysica Acta, 1726, 272–279.
Acknowledgement
The authors would like to thank CNPq (National Council of Technological and Scientific Development) for the financial support and the scholarship awarded to the first author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Knob, A., Carmona, E.C. Purification and Characterization of Two Extracellular Xylanases from Penicillium sclerotiorum: A Novel Acidophilic Xylanase. Appl Biochem Biotechnol 162, 429–443 (2010). https://doi.org/10.1007/s12010-009-8731-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-009-8731-8