Abstract
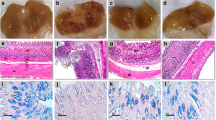
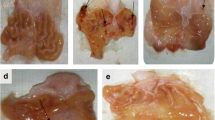
Despite the fact that dietary habits and lifestyles are incredibly advancing, gastric ulceration is still a terrible complaint. Extensive use of non-steroidal anti-inflammatory drugs (NSAIDs) and alcohol, in addition to stress, are all predisposing factors for ulcers. Most medical treatments are always time consuming and not efficient or satisfactory to the patients. Cardiovascular patients always need NSAIDs, or mostly cannot quit alcohols, while using many cardiovascular drugs. We aim to study a possible benefit of a common nitrogen oxide donor, anti-anginal drug, nicorandil [N-(2-hydroxyethyl) nicotinamide nitrate ester], in managing acute gastric ulcers through studying its effect on some relevant intermediates to ulcerogenesis as lipid peroxidation, tumor necrosis factor-alpha (TNF-α), and nitric oxide (NO). In addition, gastric mucosal histology was studied to pursue the drug effects on tissue level. Our study revealed that both indomethacin and alcohol induced gastric ulcer mainly through up-regulation of gastric mucosal lipid peroxidation, local tissue inflammation, leukocytic infiltration, and necrosis. Both ulcerogens significantly elevated TNF-α and decreased NO, initiating ulcer formation. Nicorandil pretreatment depicted a higher preventive index in indomethacin- (89.8%) and alcohol-induced (77.7%) acute ulceration. On the tissue level, it also protected the gastric mucosa combating leukocyte infiltration and tissue congestion. Nicorandil protected tissue necrosis through decreasing oxidative stress, elevating NO levels, and down-regulating the ulcerogen-induced TNF-α elevation and improved sub-mucosal blood supply. We conclude that nicorandil may be a suitable bimodal treatment for cardiovascular patients who are at high risk of gastric ulcers by using variable analgesics to alleviate possible cardiac pain episodes, and probably frequent doses will offer a more established and long-lasting protection.









Similar content being viewed by others
References
Anderson, L. E. (1994). Mosby’s medical, nursing and allied health dictionary. In K. N. Anderson, L. E. Anderson, & W. D. Glaze (p. 656). St Louis: Mosby.
Bose, M., Motghare, V. M., Dakhale, G. N., & Turankar, A. V. (2003). Antiulcer activity of levcromakalim and nicorandil in albino rats: a comparative study. Polish Journal of Pharmacology, 55, 91–95.
Giust, A. M., Raimondi, M., Ravagnan, G., Sapora, O., & Parasassi, T. (1998). Human cell membrane oxidative damage induced by single and fractional doses of ionizing radiation: a fluorescence spectroscopy study. International Journal of Radiation Biology, 74, 595–605. doi:10.1080/095530098141177.
Kwiecien, S., Brzozowski, T., Konturek, P. C., Pawlik, M. W., Pawlik, W. W., Kwiecien, N., et al. (2004). Gastroprotection by pentoxyifilline against stress-induced gastric damage. Role of lipid peroxidation, antioxidizing enzymes and proinflammatory cytokines. Journal of Physiology and Pharmacology, 55, 337–355.
Jiang, P., Chang, L., Pan, C. Z., Qi, Y. F., & Tang, C. S. (2005). Protective role of metallothionein in stress induced gastric ulcer in rats. World Journal of Gastroenterology, 11, 2739–2743.
Shian, W. M., Sasaki, I., Kamiyama, Y., Naito, H., Matsuno, S., & Miyazawa, T. (2000). The role of lipid peroxidation in gastric mucosal lesions induced by water water-immersion-restraint stress in rats. Surgery Today, 30, 40–53. doi:10.1007/PL00010046.
Wei, X. M., Heywood, G. J., Di Girolamo, N., & Thomas, P. S. (2003). Nicorandil inhibits the release of TNF alpha from a lymphocytes. International Immunopharmacology, 3, 1581–1588. doi:10.1016/S1567–5769(03)00176–0.
Mitsushige, S., Takahisa, F., Naohito, S., Akiko, N., Fang, X., Masayoshi, K., et al. (2007). Different effects of polymorphisms of tumor necrosis factor-alpha and interleukin-1 beta on development of peptic ulcer and gastric cancer. Gastroenterologia y Hepatologia, 22(1), 51–59. doi:10.1111/j.1440–1746.2006.04442.x.
Kwiecien, S., Brzozowski, T., & Konturek, S. J. (2002). Effects of reactive oxygen species on gastric mucosa in various models of mucosal injury. Journal of Physiology and Pharmacology, 53, 39–50.
Hamaguchi, M., Watanabe, T., Higuchi, K., Tominaga, K., Fujiwara, Y., & Arkawa, T. (2001). Mechanisms and roles of neutrophil infiltration in stress-induced gastric injury in rats. Digestive Diseases and Sciences, 46, 2708–2715. doi:10.1023/A:1012779530004.
Calatayud, S., Barrachina, D., & Esplugues, J. V. (2001). Nitric oxide: relation to integrity, injury and healing of the gastric mucosa. Microscopy Research and Technique, 53, 325–335. doi:10.1002/jemt.1100.
Wallace, J. L., & Miller, M. J. (2000). Nitric oxide mucosal defense: a little goes a long way. Gastroenterol, 119, 512–520. doi:10.1053/gast.2000.9304.
Fox, J. G., Back, P., Dangler, C. A., Whary, M. T., Wang, T. C., Shi, H. N., et al. (2000). Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduce Helicobacter-induced gastric atrophy. Nature Medicine, 6, 536–542. doi:10.1038/75015.
Griffin, M. R., & Scheiman, J. M. (2001). Prospects for changing the burden of non-steroidal anti-inflammatory drug toxicity. The American Journal of Medicine, 110, 33S–37S. doi:10.1016/S0002–9343(00)00634–3.
Clinch, D., Banerjee, A. K., Levy, D. W., Ostick, G., & Faragher, E. B. (1987). Non-steroidal anti-inflammatory drugs and peptic ulceration. Journal of the Royal College of Physicians of London, 21(3), 183–187.
Appleyard, C. B., McCafferty, D. M., Tigley, A. W., Swain, M. G., & Wallace, J. L. (1996). Tumor necrosis factor mediation of NSAID-induced gastric damage: role of leukocyte adherence. American Journal of Physiology. Gastrointestinal and Liver Physiology, 270, G42–G48.
Bech, P. L., Xavier, R., Lu, N., Nanda, N. N., Dinauer, M., Podolsky, D. K., et al. (2000). Mechanism of NSAID-induced gastrointestinal injury defined using mutant mice. Gastroenterol, 119, 699–705. doi:10.1053/gast.2000.16497.
Swarnakar, S., Mishra, A., Ganguly, K., & Sharma, A. V. (2007). Matrix metalloproteinase-9 activity and expression is reduced by melatonin during prevention of ethanol-induced gastric ulcer in mice. Journal of Pineal Research, 43(1), 56–64. doi:10.1111/j.1600–079X.2007.00443.x.
Khosla, P., Karan, R. S., & Bhargava, V. K. (2004). Effect of garlic oil on ethanol-induced gastric ulcers in rats. Phytotherapy Research, 18(1), 87–91. doi:10.1002/ptr.1349.
Sakai, K., Akima, M., Saito, K., Saitoh, M., & Matsubara, S. (2000). Nicorandil metabolism in rat myocardial mitochondria. Journal of Cardiovascular Pharmacology, 35, 723–728. doi:10.1097/00005344–200005000–00007.
Hedlund, P., Holmquist, F., Hedlund, H., & Andersson, K. E. (1994). Effects of nicorandil on human isolated corpus cavernosum and cavernous artery. The Journal of Urology, 151, 1107–1113.
Zhou, Q., Satake, N., & Shibata, S. (1995). The inhibitory mechanisms of nicorandil in isolated rat urinary bladder and femoral artery. European Journal of Pharmacology, 273, 153–159. doi:10.1016/0014–2999(94)00685-Z.
Patel, H. M., Santani, D. D., & Goswami, S. G. (2001). Evaluation of the effects of nicorandil on experimentally induced gastric ulcers. Pharmacol, 63, 154–159. doi:10.1159/000056127.
Toroudi, H. P., Rahgozar, M., Bakhtarian, A., & Djahanguiri, B. (1999). Potassium channel modulators and indomethacin-induced gastric ulceration in rats. Scandinavian Journal of Gastroenterology, 34, 962–966. doi:10.1080/003655299750025048.
Sakai, K., Akima, M., & Katsuyama, I. (1999). Effects of nicorandil on experimentally induced gastric ulcers in rats: a possible role of K(ATP) channels. Japanese Journal of Pharmacology, 79, 51–57. doi:10.1254/jjp.79.51.
Peskar, B. M., Ehrlich, K., & Peskar, B. A. (2002). Role of ATP-sensitive channels in prostaglandin-mediated gastroprotection in the rat. The Journal of Pharmacology and Experimental Therapeutics, 301(3), 969–974. doi:10.1124/jpet.301.3.969.
Hano, J., Bugajski, J., & Danek, L. (1976). Effect of adrenergic blockade on gastric secretion altered by catecholamines in rats. Archivum Immunologiae et Therapiae Experimentalis, 24(4), 507–524.
Mihara, M., & Uchyama, M. (1978). Determination of malondialdehyde precursor in tissues by thiobarbituric acid test. Analytical Biochemistry, 86(1), 271–278. doi:10.1016/0003–2697(78)90342–1.
de Kossodo, S., Houba, V., & Grau, G. E. (1995). Assaying tumor necrosis factor concentrations in human serum. A WHO International Collaborative study. Journal of Immunological Methods, 182(1), 107–114. doi:10.1016/0022–1759(95)00028–9.
Sastry, K. V. H., Moudgal, R. P., Mohan, J., Tyagi, J. S., & Rao, G. S. (2002). Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Analytical Biochemistry, 306(1), 79–82. doi:10.1006/abio.2002.5676.
Glavin, G. B., & Szabo, S. (1992). Experimental gastric mucosal injury: laboratory models reveal mechanisms of pathogenesis and new therapeutic strategies. The FASEB Journal, 6, 825–831.
Valcheva-Kuzmanova, S., Krasnaliev, I., Galunska, B., & Belcheva, A. (2007). Influence of DL-alpha-tocopherol acetate on indomethacin-induced gastric mucosal injury in rats. Autonomic & Autacoid Pharmacology, 27(3), 131–136. doi:10.1111/j.1474–8673.2007.00402.x.
Swarnakar, S., Ganguly, K., Kundu, P., Banerjee, A., Maity, P., & Sharma, A. V. (2005). Curcumin regulates expression and activity of matrix metalloproteinase 9 and 2 during prevention and healing of indomethacin-induced gastric ulcer. The Journal of Biological Chemistry, 280(10), 9409–9415. doi:10.1074/jbc.M413398200.
Santucci, L., Fiorucci, S., Di Matteo, F. M., & Morelli, A. (1995). Role of tumor necrosis factor alpha release and leukocyte margination in indomethacin-induced gastric injury in rats. Gastroenterology, 108(2), 393–401. doi:10.1016/0016–5085(95)90065–9.
Kunkel, S. L., Wiggins, R. C., Chensue, S. W., & Larrick, J. (1986). Regulation of macrophage tumor necrosis factor production by prostaglandin E2. Biochemical and Biophysical Research Communications, 137, 404–410. doi:10.1016/0006–291X(86)91224–6.
Hogaboam, C. M., Bissonnete, E. Y., Chin, B. C., Befus, A. D., & Wallace, J. L. (1993). Prostaglandins inhibit inflammatory mediator release from rat mast cells. Gastroenterol, 104, 122–129.
Bauer, H., Jung, T., Tsikas, D., Stichtenoth, D. O., Frolich, J. C., & Neumann, C. (1997). Nitric oxide inhibits the secretion of T-helper 1- and T-helper 2-associated cytokines in activated human T cells. Immunology, 90, 205–211. doi:10.1046/j.1365–2567.1997.00161.x.
Wallace, J. L., McKnight, W., Del Soldato, P., Baydoun, A. R., & Cirino, G. (1995). Antithrombotic effects of a nitric oxide-releasing, gastric sparing aspirin derivative. The Journal of Clinical Investigation, 96, 2711–2718. doi:10.1172/JCI118338.
Fiorucci, S., Antonelii, E., Santucci, L., Morelli, O., Miglietti, M., Federici, B., et al. (1999). Gastrointestinal safety of nitric oxide-derived aspirin is related to inhibition of ICE-like cysteine proteases in rats. Gastroenterol, 116(5), 1089–1106. doi:10.1016/S0016–5085(99)70012–0.
Fiorucci, S., Santucci, L., Gresele, P., Faccino, R. M., del Soldato, P., & Morelli, A. (2003). Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: a proof of concept endoscopic study. Gastroenterol, 124, 600–607. doi:10.1053/gast.2003.50096.
Ismail, H. A. F., Khalifa, M. M. A., Hassan, M. K., & Ashour, O. M. (2007). Investigation of the mechanisms underlying the gastroprotective effect of nicorandil. Pharmacology, 79, 76–85. doi:10.1159/000097817.
Kiefer, F., Jahn, H., Schick, M., & Wiedemann, K. (2002). Alcohol intake, tumor necrosis factor-α, leptin and craving: factors of a possibly vicious circle. Alcohol and Alcoholism (Oxford, Oxfordshire), 37(4), 401–404. doi:10.1093/alcalc/37.4.401.
Khalil, H., Ismail, H., Taye, A., & Kamel, M. (2007). Gastroprotective effect of Lippia nodiflora L. extracts in ethanol-induced gastric lesions. Pharmacognosy Magazine, 3(12), 259–262.
Duffin, R., Shaw, C. A., & Rossi, A. G. (2008). Sildenafil reduces alcohol-induced gastric damage: just say ‘NO’. British Journal of Pharmacology, 153(4), 623–624. doi:10.1038/sj.bjp.0707642.
Singh, L. P., Kundu, P., Ganguly, K., Mishra, A., & Swarnakar, S. (2007). Novel role of famotidine in down-regulation of matrix metalloproteinase 9 during protection of ethanol-induced acute gastric ulcer. Free Radical Biology & Medicine, 43(2), 289–299. doi:10.1016/j.freeradbiomed.2007.04.027.
Bhattacharya, S., Chatterjee, S., Bauri, A., Bandivdeker, A. H., Chattopadhyay, S., Bandyopadhyay, S. K., et al. (2007). Immunopharmacological basis of the healing of indomethacin-induced gastric mucosal damage in rats by the constituents of Phyllanthus emblica. Current Science, 93(1), 47–53.
Sivalingam, N., Hanumantharaya, K., Faith, M., Basivireddy, J., & Balasubramanian, K. A. (2007). Curcumin reduces indomethacin-induced damage in the rat small intestine. Journal of Applied Toxicology, 27(6), 551–560. doi:10.1002/jat.1235.
Acknowledgement
Histological examination was kindly executed by Dr. Mohamed Hamed Mohamed, Histology Department, College of Veterinary Medicine, Zagazig University, Egypt.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El-Moselhy, M.A., Abdel-Hamid, N.M. & Abdel-Raheim, S.R. Gastroprotective Effect of Nicorandil in Indomethacin and Alcohol-Induced Acute Ulcers. Appl Biochem Biotechnol 152, 449–459 (2009). https://doi.org/10.1007/s12010-008-8384-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8384-z