Abstract
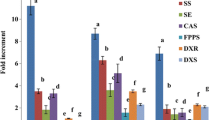
High yielding transformed callus culture of W. somnifera was established by infecting hypocotyls with Agrobacterium tumefaciens MTCC-2250. Maximum withaferin A content of 0.0875 mg/g dry cell weight and transformation efficiency of 80% were obtained. Confirmation of transformation was done on the basis of the presence of the ags gene by using polymerase chain reaction. Various abiotic elicitors (arachidonic acid, methyl jasmonate, calcium chloride, and copper sulfate) and biotic elicitors (cell extracts and culture filtrates of Alternia alternata, Fusarium solani, and Verticilium dahaliae) were tested at different concentrations to enhance withaferin A production in suspension culture of transformed cells. Maximum enhancements of 5.4 times and 9.7 times, respectively, were obtained when copper sulfate (100 μM) and the cell extract of V. dahaliae (5% v/v) were added separately to suspension cultures. The dual elicitation strategy by the combined addition of these two elicitors resulted in 13.8-fold enhancement of withaferin A content in comparison to control cultures (2.65 mg/L). The present study indicates the potential of this biotechnology-based methodology for the large-scale production of withaferin A.




Similar content being viewed by others

References
Winters, M. (2006). Alternative Medicine Review, 11, 269–277.
Al-Hindawi, M. K., Al-Khafaji, S., & Abdul-Nabi, M. (1992). Journal of Ethnopharmacology, 37, 113–116.
Devi, P. U., Sharada, A. C., Solomon, F. E., & Kamath, M. (1992). Indian Journal of Experimental Biology, 30, 169.
Bhattacharya, S. K., Satyan, K. S., & Ghosal, S. (1997). Indian Journal of Experimental Biology, 35, 236–239.
Kulkarni, S. K., George, B., & Mathur, R. (1998). Phytotherapy Research, 12, 451–453.
Furmanowa, M., Gajdzis, K. D., Ruszkowska, J., Czarnocki, Z., Obidoska, G., Sadowska, A., et al. (2001). Planta Medica, 67, 146–149.
Banerjee, S., Naqvi, A. A., Mandal, S., & Ahuja, P. S. (1994). Phytotherapy Research, 8, 452–455.
Roja, C., Heble, M. R., & Sipahimalani, A. T. (1991). Phytotherapy Research, 5, 185–187.
Vitali, G., Conte, L., & Nicoletti, M. (1996). Planta Medica, 62, 287–288.
Ray, S., & Jha, S. (1999). Plant Science, 146, 1–7.
Rani, G., & Grover, I. S. (1999). Plant Cell, Tissue and Organ Culture, 57, 23–27.
Ciddi, V. (2006). Indian Journal of Pharmaceutical Sciences, 68, 490–492.
Zhao, J., Davis, L. C., & Verpoorte, R. (2005). Biotechnology Advances, 23, 283–333.
Murashige, T., & Skoog, F. (1962). Physiologia Plantarum, 51, 473–497.
Dubois, M., GuiUes, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Analytical Chemistry, 28, 350–356.
Santiago, L. J. M., Louro, R. P., & De Oliveira, D. E. (2000). Annals of Botany, 86, 1023–1032.
Maksymiec, W., Wianowska, D., Dawidowicz, A. L., Radkiewicz, S., Mardarowicz, M., & Krupa, Z. (2005). Journal of Plant Physiology, 162, 1338–1346.
Cabello-Hurtado, F., Durst, F., Jorrin, J. S. V., & Werck-Reichhart, D. (1991). Phytochemistry, 38, 918–925.
Hakamatsuka, T., Ebizuka, Y., & Sankawa, U. (1991). Phytochemistry, 30, 1481–1482.
Gutierrez, M. C., Parry, A., Tena, M., Jorrin, J., & Edwards, R. (1995). Phytochemistry, 38, 1185–1191.
Mueller, M. J., Brodschelm, W., Spannagl, E., & Zenk, M. H. (1993). Proceedings of the National Academy of Sciences of the United States of America, 90, 7490–7494.
Wang, W., & Zhong, J. J. (2002). Journal of Bioscience and Bioengineering, 93, 48–53.
Menke, F. L. H., Parchmann, S., Mueller, M. J., Kijne, J. W., & Memelink, J. (1999). Plant Physiology, 119, 1289–1296.
Nojiri, H., Sugimori, M., Yamane, H., Nishimura, Y., Yamada, A., & Shibuya, N. (1996). Plant Physiology, 110, 387–392.
Tamogami, S., Rakwal, R., & Kodama, O. (1997). FEBS Letters, 412, 61–64.
Brader, G., Tas, E., & Palva, E. T. (2001). Plant Physiology, 126, 849–860.
Zhao, J., & Sakai, K. (2001). Journal of Experimental Botany, 54, 647–656.
Baldi, A., & Dixit, V. K. (2008). Bioresource Technology, 99, 4609–4614. DOI 10.1016/j.biortech.2007.06.061.
Preisig, C. L., & Kuc, J. A. (1985). Archives of Biochemistry and Biophysics, 236, 379–389.
Vaughn, S. F., & Lulai, E. C. (1992). Plant Science, 84, 91–98.
Hoshino, T., Chida, M., Yamaura, T., Yoshizawa, Y., & Mizutani, J. (1994). Phytochemistry, 36, 1417–1419.
Komaraiah, P., Kishor, P. B. K., Carlsson, M., Magnusson, K. E., & Mandenius, C. F. (2005). Plant Science, 168, 1337–1344.
Araceli, A. C., Elda, C. M., Edmundo, L. G., & Ernestoa, G. P. (2007). Physiological and Molecular Plant Pathology, 70, 69–76.
Sanz, K. M., Hernandez, X. E., & Tonn, C. E. (2000). Plant Cell Reports, 19, 821–824.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baldi, A., Singh, D. & Dixit, V.K. Dual Elicitation for Improved Production of Withaferin A by Cell Suspension Cultures of Withania somnifera . Appl Biochem Biotechnol 151, 556–564 (2008). https://doi.org/10.1007/s12010-008-8231-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-008-8231-2