Abstract
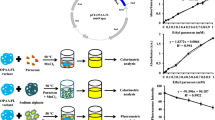
A whole cell-based amperometric biosensor for highly selective, sensitive, rapid, and cost-effective determination of the organophosphate pesticides fenitrothion and ethyl p-nitrophenol thiobenzenephosphonate (EPN) is discussed. The biosensor comprised genetically engineered p-nitrophenol (PNP)-degrading bacteria Pseudomonas putida JS444 anchoring and displaying organophosphorous hydrolase (OPH) on its cell surface as biological sensing element and carbon paste electrode as the amperometric transducer. Surface-expressed OPH catalyzed the hydrolysis of organophosphorous pesticides such as fenitrothion and EPN to release PNP and 3-methyl-4-nitrophenol, respectively, which were subsequently degraded by the enzymatic machinery of P. putida JS444 through electrochemically active intermediates to the TCA cycle. The electrooxidization current of the intermediates was measured and correlated to the concentration of organophosphates. Operating at optimum conditions, 0.086 mg dry wt of cell operating at 600 mV of applied potential (vs Ag/AgCl reference) in 50 mM citratephosphate buffer, pH 7.5, with 50 μM CoCl2 at room temperature, the biosensor measured as low as 1.4 ppb of fenitrothion and 1.6 ppb of EPN. There was no interference from phenolic compounds, carbamate pesticides, triazine herbicides, or organophosphate pesticides without nitrophenyl substituent. The service life of the biosensor and the applicability to lake water were also demonstrated.
Similar content being viewed by others
References
Beltran, J., Pitarch, E., Egea, S., Lopez, F. J., and Hernandez, F. (2001), Chromatographia 54, 757–763.
Aharonson, N., Cohen, S. Z., Drescher, N., et al. (1987), Pure Appl. Chem. 59, 1419–1446.
Wang, J., Chen, G., Muck, A., Chatrathi, M. P., Mulchandani, A., and Chen, W. (2004), Anal. Chim. Acta 505, 183–187.
Kim, D. H., Heo, G. S., and Lee, D. W. (1998), J. Chromatogr. A 824, 63–70.
Lee, X. P., Kumazawa, T., Sato, K., and Suzuki, O. (1996), Chromatographia 42, 135–140.
Schellin, M., Hauser, B., and Popp, P. (2004), J. Chromatogr. A 1040, 251–258.
Sanchez, M. E., Mendez, R., Gomez, X., and Martin-Villacorta, J. (2003), J. Liquid Chromatogr. Relat. Technol. 26, 483–497.
Cho, Y., Matsuoka, N., and Kamiya, A. (1997), Chem. Pharm. Bull. 45, 737–740.
Kolosova, A. Y., Park, J. H., Eremin, S. A., et al. (2004), Anal. Chim. Acta 511, 323–331.
Kim, Y. J., Cho, Y. A., Lee, H. S., and Lee, Y. T. (2003), Anal. Chim. Acta 494, 29–40.
Watanabe, E., Kanzaki, Y., Tokumoto, H., Hoshino, R., Kubo, H., and Nakazawa, H. (2002), J. Agric. Food Chem. 50, 53–58.
Watanabe, E., Kubo, H., and Nakazawa, H. (2002), Anal. Chim. Acta 460, 99–110.
Lui, J., Tan., M., Liang, C., and Ying, K. B. (1996), Anal. Chim. Acta 329, 297–304.
Kumaran, S. and Morita, M. (1995), Talanta 42, 649–655.
Diaz, A. N. and Peinado, M. C. R. (1997), Sens. Actuators B Chem. 39, 426–431.
Diaz, A. N., Sanchez, F. G., Bracho, V., Lovillo, J., and Aguilar, A. (1997), Fresenius J. Anal. Chem. 357, 958–961.
Sanchez, F. G., Diaz, A. N., Peinado, M. C. R., and Belledone, C. (2003), Anal. Chim. Acta 484, 45–51.
Lin, Y. H, Lu, F., and Wang, J. (2004), Electroanalysis 16, 146–149.
Donarski, W. J., Dumas, D. P., Heitmeyer, D. P., Lewis, V. E., and Raushel, F. M. (1989), Biochemistry 28, 4650–4655.
Dumas, D. P., Caldwell, S. R., Wild, J. R., and Raushel, F. M. (1989), J. Biol. Chem. 33, 19,659–19,665.
Dumas, D. P., Wild, J. R., and Raushel, F. M. (1989), Biotechnol. Appl. Biochem. 11, 235–243.
Mulchandani, A., Kaneva, I., and Chen, W. (1998), Anal. Chem. 70, 5042–5046.
Mulchandani, A., Mulchandani, P., Kaneva, I., and Chen, W. (1998), Anal. Chem. 70, 4140–4145.
Mulchandani, A., Mulchandani, P., Chen, W., Wang, J., and Chen, L. (1999), Anal. Chem. 71, 2246–2249.
Mulchandani, A., Pan, S., and Chen, W. (1999), Biotechnol. Prog. 15, 130–134.
Mulchandani, P., Mulchandani, A., Kaneva, I., and Chen, W. (1999), Biosens. Bioelectron. 14, 77–85.
Mulchandani, A., Chen, W., Mulchandani, P., Wang, J., and Rogers, K. R. (2001), Biosens. Bioelectron. 16, 225–230.
Mulchandani, P., Chen, W., and Mulchandani, A. (2001), Environ. Sci. Technol. 35, 2562–2565.
Mulchandani, P., Chen, W., Mulchandani, A., Wang, J., and Chen, L. (2001), Biosens. Bioelectron. 16, 433–437.
Rogers, K. R., Wang, Y., Mulchandani, A., Mulchandani, P., and Chen, W. (1999), Biotechnol. Prog. 15, 517–522.
Wang, J., Chen, L., Mulchandani, A., Mulchandani, P., and Chen, W. (1999), Electroanalysis 11, 866–869.
Chough, S. H., Mulchandani, A., Mulchandani, P., Chen, W., Wang, J., and Rogers, K. R. (2002), Electroanalysis 14, 273–276.
Wang, J., Chatrathi, M. P., Mulchandani, A., and Chen, W. (2001), Anal. Chem. 73, 1804–1808.
Lei, Y., Mulchandani, P., Chen, W., Wang, J., and Mulchandani, A. (2004), Biotechnol. Bioeng. 15, 706–713.
Lei, Y., Mulchandani, A., and Chen, W. (2005), Biotechnol. Prog. 21, 678–681.
Lei, Y., Mulchandani, P., Chen, W., and Mulchandani, A. (2005), J. Agric. Food Chem. 53, 524–527.
Lei, Y., Mulchandani, P., Chen, W., and Mulchandani, A. (2006), Sensors 6, 466–472.
Lei, Y., Mulchandani, P., Chen, W., and Mulchandani, A. (2005), Environ. Sci. Technol. 39, 8853–8857.
Han, T. S., Kim, Y. C., and Karube, I. (2001), Anal. Chim. Acta 431, 225–230.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lei, Y., Mulchandani, P., Chen, W. et al. Biosensor for direct determination of fenitrothion and EPN using recombinant Pseudomonas putida JS444 with surface-expressed organophosphorous hydrolase. 2. Modified carbon paste electrode. Appl Biochem Biotechnol 136, 243–250 (2007). https://doi.org/10.1007/s12010-007-9023-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s12010-007-9023-9