Abstract
Background
Computer-assisted surgery (CAS) for cup placement has been developed to improve the functional results and to reduce the dislocation rate and wear after total hip arthroplasty (THA). Previously published studies demonstrated radiographic benefits of CAS in terms of implant position, but whether these improvements result in clinically important differences that patients might perceive remains largely unknown.
Questions/purposes
We hypothesized that THA performed with CAS would improve 10-year patient-reported outcomes measured by validated scoring tools, reduce acetabular polyethylene wear as measured using a validated radiological method, and increase survivorship.
Methods
Sixty patients operated on for a THA between April 2004 and April 2005 were randomized into two groups using either the CAS technique or a conventional technique for cup placement. All patient candidates for a THA with the diagnosis of primary arthritis or avascular necrosis were eligible for the CAS procedure and randomly assigned to the CAS group by the Hospital Informatics Department with use of a systematic sampling method. The patients assigned to the freehand cup placement group were matched for sex, age within 5 years, pathological condition, operatively treated side, and body mass index within 3 points. All patients were operated on through an anterolateral approach (patient in the supine position) using cementless implants. In the CAS group, a specific surgical procedure using an imageless cup positioning computer-based navigation system was performed. There were 16 men and 14 women in each group; mean age was 62 years (range, 24–80 years), and mean body mass index was 25 ± 3 kg/m2. No patient was lost to followup at 10 years, but five patients have died (two in the CAS group and three in the control group). At the 10-year followup, an independent observer blinded to the type of technique performed patients’ evaluation. Cup positioning was evaluated postoperatively using a CT scan in the two groups with results previously published. At 10 years, we assessed subjective functional outcome and quality of life using validated questionnaires (SF-12, Harris hip score [HHS], Hip injury and Osteoarthritis Outcome Score). Wear rate was then evaluated on standardized radiographs using a previously validated semiautomated computer analogic measurement method (dual circle method). Complications and survivorship were compared between groups. With our available sample size, this study had 80% power to detect a difference of 4 points out of 100 on the HHS at the p < 0.05 level.
Results
With the numbers available, we found we found no differences between groups regarding HSS at last followup 95.3 ± 5.9 points (CAS group) versus 96.2 ± 4.5 points, a mean difference of 0.9 points (95% confidence interval [CI], −4.3 to 4.6; p = 0.6). There was no difference between the groups in terms of the mean (± SD) acetabular linear wear at 10 years. The mean wear was 0.71 ± 0.6 mm in the CAS group versus 0.77 ± 0.52 mm in the control group, a mean difference of 0.06 mm (95% CI, −0.1 to 0.2; p = 0.54). With the numbers available, there was no difference between the CAS group and the conventional THA groups in terms of survivorship free from aseptic loosening (100%; 95% CI, 100%–95%, versus 100%; 95% CI, 100%–94%; p = 0.3).
Conclusions
Our observations suggest that CAS used for cup placement does not confer any substantial advantage in function, wear rate, or survivorship at 10 years after THA. Because CAS is associated with added costs and surgical time, future studies need to identify what clinically relevant advantages it offers, if any, to justify its continued use in THA.
Level of Evidence
Level II, therapeutic study.
Similar content being viewed by others
Introduction
Improper acetabular component positioning during THA may increase the risk of dislocation, reduce the ROM free from intraarticular impingement, and cause increased acetabular wear [1–5, 8, 10]. There have been numerous reports regarding the optimal orientation of the acetabular component in THA [1–5, 8]. Although debate remains on this topic, the classical target for cup orientation in THA is the so-called “Lewinnek safe zone” with an abduction angle of 40° ± 10° and an anteversion angle of 15° ± 10° [1–5, 8]. Use of mechanical acetabular guides for intraoperative alignment leads to variations between the actual and desired implant orientation mainly as a result of the variations of the patient position on the operative table [5]. To limit the potential cup malposition, computer-assisted orthopaedic navigation systems were developed in the early 2000s. After initial adoption and demonstration of their usefulness to improve the reliability of cup placement [5, 6, 18], standard computer-assisted systems have been improved to limit the extra time required for the procedure. The latest evolution of computer-assisted surgery (CAS) for THA has been recently introduced based on systems using robotic-assisted computer navigation. The concept behind this technology is that the robot assists the user to follow the navigated plan for cup positioning, and the guided process could result in potentially more accurate reaming [6]. In a previous prospective comparative randomized study, we demonstrated the usefulness of CAS in THA to improve cup positioning [18].
However, to our knowledge, few studies have looked at CAS over the longer term to see whether it confers any advantages in terms of endpoints that a patient might perceive such as differences in validated outcome scores, differences in acetabular wear (which can lead to osteolysis or revision surgery), or differences in the proportion of patients undergoing revision surgery [11, 21, 23]. Therefore and as a result of the resurgence of interest for computer-assisted cup positioning in THA, as a followup of this prospective case-matching cohort study, we aimed to compare the 10-year results between patients undergoing THA with CAS and those undergoing THA with a conventional technique for component implantation. Specifically, we compared the groups in terms of (1) validated outcomes scores (Harris hip score [HHS], Hip injury and Osteoarthritis Outcome Score [HOOS], and SF-12); (2) acetabular polyethylene wear as measured using a validated in vivo radiographic method; and (3) survivorship free from aseptic loosening.
Patients and Methods
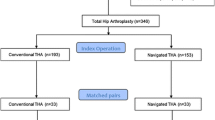
From April 2004 to April 2005, we performed a prospective case-matching cohort study including two groups of 30 patients. Between April 2004 and April 2005, we performed 260 elective THAs for primary arthritis or avascular osteonecrosis. Of those, 30 (12%) were performed using the CAS. During that period, we used CAS for the patients meeting the inclusion criteria of our study and randomly assigned to the CAS group by the Hospital Informatics Department with use of a systematic sampling method. Of those who were treated with this approach, two patients (6%) had died, and no patients were lost to followup, whereas 28 patients (28 hips [94%]) were available for followup at a minimum of 10 years (median, 10 years; range, 10–11 years). The study protocol (including the use of navigation and postoperative CT evaluation) and consent forms were approved by the local ethical committee. The patient inclusion criteria were an age of 20 to 80 years old, a weight of < 100 kg, a primary hip arthroplasty, an anterolateral approach, and procedure performed by the senior author (J-NAA). The exclusion criteria included use of a trochanteric osteotomy, THA performed for a posttraumatic indication, or revision hip surgery. Randomization of patients into the computer-assisted group was done by the Hospital Informatics Department with use of a systematic sampling method according to a previously described method. All of the patients provided informed consent to participate in the study. The patients assigned to the freehand cup placement group were matched for sex, age within 5 years, pathological condition, operatively treated side, and body mass index within 3 points (Fig. 1). Traditional mechanical guides were used in the freehand placement group. Before the beginning of the study, the senior author had performed more than 2000 THAs, which included 50 computer-assisted procedures.
There were no differences in demographic data between the two study groups with the numbers available. There were 16 men and 14 women and 14 right hips and 16 left hips in each group. The median age of the patients was 60 years (range, 24–80 years) in the computer-assisted group and 61 (range, 26–78 years) in the freehand placement group (p = 0.29). The mean body mass index was 26 ± 5 kg/m2 (range, 17–37 kg/m2) in the computer-assisted group and 25 ± 4 kg/m2 (range, 20–38 kg/m2) in the freehand placement group (p = 0.28). The etiologies were primary osteoarthritis in 27 hips and osteonecrosis in three hips in the computer-assisted group and primary osteoarthritis in 26 hips and osteonecrosis in four hips in the freehand placement group.
The operation was done through a modified Watson-Jones anterolateral approach with the patient lying supine. Patient position allowed palpation of both anterosuperior iliac spines and the symphysis pubis. No additional skin incision had to be made to accommodate navigation. In the control group, manual positioning of the cup was performed. In the CAS group, an imageless cup positioning computer-based navigation system was used following a previously described protocol [18]. A cementless press-fit hydroxyapatite-coated titanium acetabular component with a conventional (not highly crosslinked) UHWPE (sterilization with gamma radiation under nitrogen) and a cementless fully hydroxyapatite-coated titanium femoral stem were used (Symbios®, Yverdon, Switzerland, FDA-approved) in the two groups. The stems were not navigated and were inserted with a nonspecific fixed angle of anteversion according to surgeon judgment. A 28-mm ceramic head was systematically used in both groups. The median diameter of the acetabular cup was 50 mm (range, 46–58 mm) in the CAS group and 52 mm (range, 46–58 mm) in the freehand placement group. Postoperative rehabilitation protocols included immediate weightbearing protected by crutches during the first 2 or 3 weeks according to patient tolerance and exercises focused on passive ROM immediately and then active recuperation of ROM. All patients in the present study received routine prophylaxis with low-molecular-weight heparin pre- and postoperatively for 21 days.
All patients were evaluated clinically preoperatively, at 3 months postoperatively, and at yearly intervals postoperatively by one of the authors (SP). At the 10-year followup, the evaluation was performed by two independent observers blinded to the type of technique (MO, AL). Pre- and postoperatively we obtained a HHS to objectively determine the patient’s functional level. At last followup, the HOOS was used to evaluate the patient’s hip-related quality of life [17] and the SF-12 to evaluate the patient’s general health-related quality of life [22]. The HOOS is a self-administrated hip-related quality-of-life questionnaire corresponding to a validated and improved WOMAC [23]. The HOOS includes five dimensions scored separately: pain (nine items); symptoms (seven items); activities of daily life function (17 items); sport and recreation function (five items); and quality of life (four items). Because it is desirable to analyze and interpret the five dimensions separately, an aggregate score was not calculated. All items are scored from 0 to 4, and each of the five scores is calculated as the sum of the items included. Scores are then transformed using free calculation software available online on the website www.koos.nu to a 0 to 100 scale with zero representing extreme hip problems and 100 representing no hip problems. In this study the HOOS and the SF-12 scores were not calculated preoperatively because they were not available in our country.
Postoperatively, cup positioning was analyzed using a validated specific CT scan protocol. As published by Lewinnek et al. [12], the safe zone for cup orientation was defined by an abduction angle of 40° ± 10° and an anteversion angle of 15° ± 10°. Acetabular components were described as inside or outside of the safe zone or outliers based on this definition.
For the followup, radiographic postoperative evaluation consisted of AP and lateral views of the hip and pelvis and a true lateral view of the hip. The first postoperative radiograph was then used as a baseline from which subsequent radiographs were interpreted. At last followup, the radiographs were digitalized with a high-density scanner (SIERRA Advantage VIDAR Systems Corporation®, Herndon, VA, USA) and examined by two independent observers (MO, AL). Magnification correction factors were calculated for each film based on the known diameter of the prosthetic head. Polyethylene wear was measured using IMAGIKA® software (GSI Medical, Neuilly sur Seine, France), data processing procedures based on the dual circle method to analyze digitalized radiographs. This software has already proved its reproducibility and accuracy [7]. The examiners analyzed each radiograph twice, once on each of 2 separate days according to a previously published protocol [16]. All data processing was performed independently from one another. The total wear was compared as well as wear rate per year, and this linear wear was expressed as mean ± SD (millimeters per year).
Survivorship was compared in the two groups using the Kaplan-Meier method [9] defining the endpoint as: dislocation, revision of one or both of the components resulting from a mechanical failure, or mechanical failure (fracture excepted) defined as substantial migration of one or both of the components, substantial wear, or osteolysis at last followup potentially calling for a revision. Loosening was analyzed using the following criteria for the femur and the acetabular cup: acetabular cup stability was evaluated with the Massin et al. method analyzing the distance between femoral head center and landmark along a vertical axis (the distance between the center of the cup and the teardrop line) and horizontal axis (distance between the center of the cup and the vertical line through the teardrop) [15]. A difference between the postoperative value and at last followup, greater than 3°, was considered as migration. The tilt of the acetabular component (alpha angle) was defined as the angle between the cup and the teardrop line. A variation greater than 3° between initial and followup alpha angle was interpreted as migration. Radiolucent lines in the DeLee and Charnley zone were analyzed and interpreted as important depending of their size, localization, and evolution [4].
Statistical Analysis
Clinical improvement between the preoperative and the last followup clinical evaluation (HHS) was compared between the CAS group and the control group and between the cups within the safe zone and the outliers using a t-test for unpaired comparisons. A comparison of the HOOS and SF-12 at last followup between CAS versus control patients as well as safe zone versus outlier patients was performed using either a parametric or nonparametric test (depending on parameter distribution). With our available sample size and postoperative score SD, this study had 80% power to detect a difference of 4 points out of 100 on the HHS at the p < 0.05 level. Because the minimal clinical important difference (MCID) of the HHS score has been described to be 10 points [20], our sample size was sufficient to detect potential relevant differences between the CAS and control groups regarding this clinical parameter. We compared survivorship in the two groups using the method of Kaplan-Meier using the previously described criteria as the endpoint [9]. Revisions related to septic loosening or fractures were not included in the survivorship comparison.
Analysis was performed using SPSS software (Version 12; SPSS Inc, Chicago, IL, USA). All calculations assumed two-tailed tests.
Results
No differences with the numbers available were found between the two groups for the HHS, SF-12, or HOOS (Fig. 2) at last followup (Table 1). HHS increased in both groups from preoperative to last followup evaluation with, respectively, in the CAS group 62 ± 10 to 95 ± 6 (p < 0.0001) and 57 ± 16 to 96 ± 5 (p < 0.0001) for control patients (Table 1). At last followup the HHS score was 95.3 ± 5.9 points (CAS group) versus 96.2 ± 4.5 points, a mean difference of 0.9 points (95% confidence interval [CI], −4.3 to 4.6; p = 0.6).
Ten-year HOOS analysis for (A) CAS versus control group; and (B) safe zone versus outliers are shown. (A) No statistical difference was found when comparing functional outcomes of CAS and control groups. (B) No statistical difference was found when comparing functional outcomes of safe zone and outlier patients. ADL = activities of daily living; QOL = quality of life.
There was no difference, with the numbers available, in wear between groups. The amount of wear at 10 years was not different with the numbers available in the two groups: 0.71 ± 0.6 mm (CAS group) versus 0.77 ± 0.52 mm (control group), a mean difference of 0.06 mm (95% CI, (−0.1 to 0.2; p = 0.54) as well as linear wear: 0.07 ± 0.06 mm/year (CAS group) versus 0.07 ± 0.05 mm/year (control group), a mean difference of 0.007 mm (95% CI, −0.02 to 0.02; p = 0.45). Radiological analysis did not exhibit any difference between CAS versus control patients as well as between the safe zone versus outlier patients regarding osteolysis, implant migration, or “cup tilt” variation (Tables 2, 3, 4).
With the numbers available, there was no difference between the CAS group and the conventional THA groups in terms of survivorship free from aseptic loosening (100%; 95% CI, 100%–95% versus 100%; 95% CI, 100%–94%; p = 0.3; Fig. 3). No dislocation was observed in the series. In the control group, one patient underwent reoperation 7 years after a periprosthetic fracture.
Discussion
Improper acetabular component during THA may increase the risk of dislocation, reduce the ROM free of intraarticular impingement, and cause increased acetabular wear. Recently, CAS for THA has been reintroduced using robotic-assisted computer navigation [6]. In a previous prospective comparative randomized study, we demonstrated the usefulness of CAS in THA to improve cup positioning [18]. However, to our knowledge, few studies have looked at CAS over the longer term to see whether it confers any advantages in terms of endpoints that a patient might perceive such as differences in validated outcome scores, differences in acetabular wear (which can lead to osteolysis or revision surgery), or differences in the proportion of patients undergoing revision surgery [11, 21, 23]. As a result of the resurgence of interest for computer-assisted cup positioning in THA, as a followup of this prospective, case-matching cohort study, we aimed to compare the 10-year results between the two groups of patients. The results of our study showed no differences in validated outcomes scores, acetabular wear, or survivorship at 10 years.
Several limitations can be outlined in our study. The first issue is sample size. As a result of the small sample size, the comparison between the safe zone and the outlier patients was not possible. Although our study was relatively small, we had 80% power to detect a difference between the treatment groups of 4 points for the HSS score, 10 points for the SF-12, and 15 points for the HOOS. Second, the wear rate was analyzed on standard radiographs and not on CT scan with a standardization of the radiographs during the followup. The method used in our study has, however, been previously validated [7]. Third, the target used in this study was based on the Lewinnek et al. [12] study and a recent paper showed that this target may not be appropriate [1], although most of the available systems today still use this target [6, 23]. Concerning the surgical technique, the system was strictly a cup positioning software with no integration of the combined cup-stem anteversion. Furthermore, the patients over 100 kg were not included in the study. These limits are mainly related to the fact that the system used in our study called anatomical navigation and based on the acquisition of the anterior pelvic plane was not validated for obese patients and for the combined anteversion at the time of the original study [18].
At 10 years, the clinical outcomes including the HSS, the HOOS, and the SF-12 were comparable in the two groups. In two previous studies (comparison of limb length discrepancy after THA: with and without computer navigation), no clinical difference was observed for the HHS or the Postel-Merle score between the CAS group and standard groups at, respectively, 1 year and 13 years [13, 21]. To our knowledge, only one previous study used the HOOS as a specific hip quality-of-life questionnaire to compare the two techniques with no difference observed between the two groups at 5 to 7 years after surgery. The authors of this article concluded that “standard cup first” THA navigation does not improve midterm functional outcome [11]. If most of the studies including the recent meta-analysis comparing CAS THA versus a conventional technique reported improvement in cup positioning, there is still a lack of evidence to link this cup position improvement to substantial clinical improvements at short and midterm followup [23]. Based on our results at long term, and based on the analysis of the work of others as noted, there is no evidence at short or midterm followup showing a potential functional benefit when using CAS for cup positioning.
The wear rates were not different with the numbers available between the two groups in our study. At 5 to 7 years followup, Keshmiri et al. [11] likewise reported no differences between navigated and conventional THAs in terms of acetabular wear. Direct comparison of our results is not possible because the followup was different and a 32-mm metal head was used in the Keshmiri et al. [11] study, whereas a 28-mm ceramic head was used in our study, but in both studies, no difference between CAS and the standard group was observed. At 13 years followup, Sugano et al. using ceramic-on-ceramic components reported a higher rate of femoral neck erosion on the lateral radiographs in the standard group compared with the navigated group [21]. Careful placement of the acetabular component to ensure an acetabular angle less than 45° in the reconstructed hip has been shown as a factor to reduce wear in conventional polyethylene [14]. However, many factors may influence polyethylene wear including the reproduction of the femoral offset and also factors directly related to the patient such as the degree of activity, age, and body mass index. In our study, the femoral offset was not calculated and the degree of activity was not recorded, but age and body mass index were comparable between the two groups.
In our study, survivorship was compared in the two groups using the Kaplan-Meier method considering the endpoint as: dislocation, revision of one or both of the components resulting from a mechanical failure, or mechanical failure defined as substantial migration of one or both of the components, substantial wear, or osteolysis at last followup potentially calling for a revision. No difference was observed between the two groups at 10 years. To our knowledge, only one study has compared the survivorship between CAS THA and standard THA [21]. Sugano et al. found no difference at 13 years between the CAS group and the standard group and no dislocation was observed in the series [21]. One of the first premises of CAS THA was to reduce the rate of dislocation because of improved cup positioning within the classical so-called safe zone. The results of our study did not show any difference between the two groups in terms of dislocation, although with such small numbers, one might not necessarily expect to observe a difference. No paper to our knowledge has demonstrated any difference between CAS and the standard technique concerning the risk of dislocation. A recent paper [1] showed that the actual targeted values for cup inclination and anteversion based on the original Lewinnek et al. [12] paper may be useful but should not be considered as a safe zone in the sense that positioning the acetabular component within these parameters does not preclude dislocation. In fact, the majority of dislocated THAs were within the targeted values.
To our knowledge, this study is the first prospective comparative case-matching cohort study comparing the 10-year clinical and radiological results including the survivorship of CAS cup positioning versus manual positioning. No difference was observed at 10 years in this prospective randomized study comparing the functional outcomes, the wear rate, and the survivorship of CAS for cup positioning in THA versus the standard technique at 10 years. Although CAS using either navigation or robotics is an accurate tool to position the cup within targets as historically defined, questions remain concerning the relevance of these targets [1, 19]. The robotic era of THA should take into account these actual limitations and further studies are required to integrate the static and dynamic parameters to define an individual optimal component positioning in THA. Because CAS is associated with added costs and surgical time, future studies need to identify what clinically relevant advantages it offers, if any, to justify its continued use in THA.
References
Abdel MP, von Roth P, Jennings MT, Hanssen AD, Pagnano MW. What safe zone? The vast majority of dislocated THAs are within the Lewinnek safe zone for acetabular component position. Clin Orthop Relat Res. 2015 Jul 7 [Epub ahead of print].
Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stockl B. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br. 2005;87:762–769.
D’Lima DD, Urquhart AG, Buehler KO, Walker RH, Colwell CW Jr. The effect of the orientation of the acetabular and femoral components on the range of motion of the hip at different head-neck ratios. J Bone Joint Surg Am. 2000;82:315–321.
DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32.
Digioia AM 3rd, Jaramaz B, Plakseychuk AY, Moody JE Jr, Nikou C, Labarca RS, Levison TJ, Picard F. Comparison of a mechanical acetabular alignment guide with computer placement of the socket. J Arthroplasty. 2002;17:359–364.
Domb BG, El Bitar YF, Sadik AY, Stake CE, Botser IB. Comparison of robotic-assisted and conventional acetabular cup placement in THA: a matched-pair controlled study. Clin Orthop Relat Res. 2014;472:329–336.
Girard J, Touraine D, Soenen M, Massin P, Laffargue P, Migaud H. Measurement of head penetration on digitalized radiographs: reproducibility and accuracy. Rev Chir Orthop Reparatrice Appar Mot. 2005;91:137–142.
Jolles BM, Zangger P, Leyvraz PF. Factors predisposing to dislocation after primary total hip arthroplasty: a multivariate analysis. J Arthroplasty. 2002;17:282–288.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481.
Kennedy JG, Rogers WB, Soffe KE, Sullivan RJ, Griffen DG, Sheehan LJ. Effect of acetabular component orientation on recurrent dislocation, pelvic osteolysis, polyethylene wear, and component migration. J Arthroplasty. 1998;13:530–534.
Keshmiri A, Schröter C, Weber M, Craiovan B, Grifka J, Renkawitz T. No difference in clinical outcome, bone density and polyethylene wear 5-7 years after standard navigated vs conventional cementfree total hip arthroplasty. Arch Orthop Trauma Surg. 2015;135:723–730.
Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220.
Licini DJ, Burnikel DJ, Meneghini RM, Ochsner JL. Comparison of limb-length discrepancy after THA: with and without computer navigation. Orthopedics. 2013;36:e543–547.
Little NJ, Busch CA, Gallagher JA, Rorabeck CH, Bourne RB. Acetabular polyethylene wear and acetabular inclination and femoral offset. Clin Orthop Relat Res. 2009;467:2895–2900.
Massin P, Schmidt L, Engh CA. Evaluation of cementless acetabular component migration. An experimental study. J Arthroplasty. 1989;4:245–251.
Ollivier M, Frey S, Parratte S, Flecher X, Argenson JN. Does impact sport activity influence total hip arthroplasty durability? Clin Orthop Relat Res. 2012;470:3060–3066.
Ornetti P, Parratte S, Gossec L, Tavernier C, Argenson JN, Roos EM, Guillemin F, Maillefert JF. Cross-cultural adaptation and validation of the French version of the Hip disability and Osteoarthritis Outcome Score (HOOS) in hip osteoarthritis patients. Osteoarthritis Cartilage. 2010;18:522–529.
Parratte S, Argenson JN. Validation and usefulness of a computer-assisted cup-positioning system in total hip arthroplasty. A prospective, randomized, controlled study. J Bone Joint Surg Am. 2007;89:494–499.
Parratte S, Pagnano MW, Coleman-Wood K, Kaufman KR, Berry DJ. The 2008 Frank Stinchfield award: variation in postoperative pelvic tilt may confound the accuracy of hip navigation systems. Clin Orthop Relat Res. 2009;467:43–49.
Shi H-Y, Chang J-K, Wong C-Y, Wang J-W, Tu Y-K, Chiu H-C, Lee K-T. Responsiveness and minimal important differences after revision total hip arthroplasty. BMC Musculoskelet Disord. 2010;11:261.
Sugano N, Takao M, Sakai T, Nishii T, Miki H. Does CT-based navigation improve the long-term survival in ceramic-on-ceramic THA? Clin Orthop Relat Res. 2012;470:3054–3059.
Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233.
Xu K, Li YM, Zhang HF, Wang CG, Xu YQ, Li ZJ. Computer navigation in total hip arthroplasty: a meta-analysis of randomized controlled trials. Int J Surg. 2014;12:528–533.
Acknowledgments
We thank Vincent Pradel MD, PhD, and Vanessa Pauly PhD, from the statistical department of the hospital for their help during the allocation of the patients in the CAS group.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he, or a member of his immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Institute for Locomotion, Aix-Marseille University, Marseille, France.
About this article
Cite this article
Parratte, S., Ollivier, M., Lunebourg, A. et al. No Benefit After THA Performed With Computer-assisted Cup Placement: 10-year Results of a Randomized Controlled Study. Clin Orthop Relat Res 474, 2085–2093 (2016). https://doi.org/10.1007/s11999-016-4863-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-016-4863-7