Abstract
Background
Resection of diaphyseal bone tumors for local tumor control and stabilization often results in an intercalary skeletal defect and presents a reconstructive challenge for orthopaedic surgeons. Although many options for reconstruction have been described, relatively few studies report on the functional outcomes and complications of patients treated with modular intercalary endoprostheses.
Questions/purposes
The objectives of this study were to examine clinical outcomes after reconstruction with a modular intercalary endoprosthesis with a specific focus on (1) the rate of complication or failure; (2) differences in complication rates by anatomic site; (3) functional results as assessed by the Musculoskeletal Tumor Society System (MSTS); and (4) differences in complication rate between patients treated with cemented versus noncemented fixation.
Methods
We conducted a retrospective chart review of patients treated with a modular intercalary endoprosthesis from three musculoskeletal oncology centers from 2008 to 2013. The indication for use of this intercalary endoprosthesis was segmental bone loss from aggressive or malignant tumor with sparing of the joint above and below and deemed unsuitable for biologic reconstruction. No other implant was used for this indication during this period. During this period, 41 patients received a total of 44 intercalary implants, which included 18 (40%) humeri, 5 (11%) tibiae, and 21 (48%) femora. There were 27 (66%) men and 14 (34%) women with a mean age of 63 years (range, 18–91 years). Eight patients (20%) had primary bone tumors and 33 (80%) had metastatic lesions. Thirty-five (85%) patients were being operated on as an initial treatment and six (15%) for revision of a previous reconstruction. Twenty-nine (66%) procedures had cemented stem fixation and 15 (34%) were treated with noncemented fixation. The overall mean followup was 14 months (range, 1–51 months). Patients with primary tumors had a mean followup of 19 months (range, 4–48 months) and patients with metastatic disease had a mean followup of 11 months (range, 1–51 months). Causes of implant failure were categorized according to Henderson et al. [19] into five types as follows: Type I (soft tissue failure), Type II (aseptic loosening), Type III (structural failure), Type IV (infection), and Type V (tumor progression). At 2 years of followup, 38 (93%) of these patients were accounted for with three (7%) lost to followup. MSTS functional assessment was available for 39 of 41 patients (95%).
Results
At latest followup of these 41 patients, 14 (34%) patients were dead of disease, two patients (5%) dead of other causes, seven (17%) are continuously disease-free, one (2%) shows no evidence of disease, and 17 (41%) are alive with disease. There were 12 (27%) nononcologic complications. Five (11%) of these were Type II failures occurring in noncemented implants between the stem and bone, and six (14%) were Type III failures occurring in cemented implants at the clamp-rod implant interface. One patient developed a deep infection (2%, Type IV failure) and underwent removal of the implant. Additionally, one patient (2%, Type V failure) was treated by amputation after local progression of his metastatic disease. Complications were more common in femoral reconstructions than in tibial or humeral reconstructions. Twelve of 21 patients (57%) with femoral reconstructions had complications versus 0% of tibial or humeral reconstructions (0 of 23; odds ratio [OR], 62; 95% confidence interval [CI], 3–1154; p < 0.0001). The mean overall MSTS score was 77%. Implants with cemented fixation (29) had higher mean MSTS scores when compared with implants with noncemented (15) fixation (84% versus 66%, p = 0.0017). The complication rate was 33% in noncemented cases and 21% in cemented cases (p = 0.39); however, Type II failure at the bone-stem interface was associated with noncemented fixation and Type III failure at the clamp-rod interface was associated with cemented fixation (OR, 143; 95% CI, 2.413–8476; p = 0.0022).
Conclusions
The results of this study indicate that this modular intercalary endoprosthesis yields equivalent results to other studies of intercalary endoprostheses in terms of MSTS scores. We found that patients treated with intercalary endoprostheses in the femur experienced more frequent complications than those treated for lesions in either the humerus or tibia and that the femoral complication rate of this endoprosthesis is higher when compared with other studies of intercalary endoprostheses for femoral reconstruction. Further studies are still needed to determine the long-term outcomes of this endoprosthesis in patients with primary tumors where longevity of the implant is of more importance than in the metastatic setting. We recommend cemented fixation for this intercalary modular endoprostheses because this provides improved MSTS scores and allows immediate return to weightbearing, which is of advantage to metastatic patients with limited lifespans.
Level of Evidence
Level III, therapeutic study.
Similar content being viewed by others
Introduction
Treatment of diaphyseal bone tumors may involve resection for local tumor control and for skeletal stabilization [35]. With advances in imaging and treatment, joint-sparing procedures have allowed more frequent use of intercalary resection [3, 28, 30, 34] . Segmental resection of the diaphysis of a long bone allows preservation of joint function and in skeletally immature patients allows for preservation of the physes. Many surgical options for the resection, reconstruction, and stabilization of segmental intercalary defects have been described and include the biologic options of autografts [7, 8, 29], allografts [5, 6, 11, 12, 15, 22, 23, 25, 26], and distraction osteogenesis [13, 36] or use of metal constructs including segmental intercalary endoprostheses [9, 10, 31] and, although less practical, custom implants [1, 2, 4, 18, 24, 33]. There have been few reports that analyze functional outcomes after treatment with modular segmental intercalary endoprostheses.
The aim of this study is to report the outcomes and complications in patients treated with an intramedullary diaphyseal segmental defect fixation system (IDSF) from three musculoskeletal oncology centers (Rutgers New Jersey Medical School, Newark, NJ, USA; University Clinic of Bonn, Bonn, Germany; University Medical Center Schleswig-Holstein, Campus Lübeck, Lübeck, Germany). In this study, we asked the following questions: (1) What proportion of patients experience a complication or a failure of intercalary endoprostheses used to reconstruct a diaphyseal resection? (2) Do the complications vary with anatomic site? (3) What are the functional results of intercalary endoprostheses as assessed by the Musculoskeletal Tumor Society System? (4) Are there differences in outcome scores or the risk of complications between patients treated with cemented versus noncemented fixation?
Patients and Methods
We retrospectively studied the records at three musculoskeletal oncology centers from 2008 to 2013. Inclusion criteria were patients with segmental bone loss from an aggressive or malignant tumor with sparing of the joint above and below and deemed unsuitable for biologic reconstruction who were surgically treated with a modular intercalary endoprosthesis (OsteoBridge™ IDSF; Merete, Berlin, Germany) in the diaphysis of the humerus, tibia, or femur. These included patients with skeletal defects ≥ 4 cm in the humerus and ≥ 5 cm in the tibia and femur. During this period this was the only implant used for this indication. We identified 41 patients (44 implants) with diaphyseal and metadiaphyseal defects after resection. We recorded the age and gender of the patients; the indication for surgery; the size, location, and histopathology of the tumor as well as the date of surgery, followup, and complications. The resection length as well as the diameter and length of both proximal and distal stems was recorded in addition to the method of fixation.
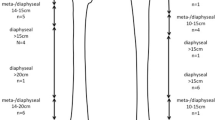
Endoprosthetic reconstruction was performed 44 times in 41 patients, which included 18 (40%) humeri, five (11%) tibiae, and 21 (48%) femora. There were 27 (66%) men and 14 (34%) women. Histologic diagnosis as well as patient age, surgical details, followup, complications, and MSTS subscores and total scores for each patient is reported (Table 1). Eight patients (20%) with a mean age of 49 years (range, 18–74 years) had primary tumors and 33 (80%) patients with a mean age of 68 years (range, 46–91 years) had metastatic lesions. At latest followup of these 41 patients, 14 (34%) patients were dead of disease (DOD), two patients (5%) dead of other causes, seven (17%) are continuously disease-free, one (2%) shows no evidences of disease, and 17 (41%) are alive with disease. In 38 patients (86%), surgery was indicated as an initial treatment of a tumor. Six patients (14%) were operated on for revision of a previous failed limb preservation surgery. Of these, one patient underwent resection of their metastatic breast cancer and implantation of the IDSF endoprosthesis (Patient 15) and five patients had failed previous reconstructions (two allograft fractures: Patients 30 and 40a, two failed intramedullary nails: Patients 29 and 37, and one failed endoprosthesis [MUTARS®; Implantcast, Buxtehude, Germany]: Patient 20). Additionally, one patient with metastatic renal cell carcinoma in the humerus developed a second metastatic site in the femur (Patient 22b) and two patients had a second complication after revision of the original endoprosthesis as a result of spacer clamp failure (Patients 26b and 40b). The mean defect size was 9 cm (humerus = 6 cm, tibia = 11 cm, femur = 9 cm). The mean proximal stem length was 11 cm (range, 1–20 cm) with a mean diameter of 12 mm (range, 7–16 mm). The mean distal stem length was 9 cm (range, 1–20 cm) with a mean diameter of 12 mm (range, 7–16 mm). The prosthesis was fixed with polymethylmethacrylate (PMMA) in 29 (66%) cases and 15 (34%) prostheses were noncemented. The average time from surgery to the development of a complication was 14 months (range, 1–50 months) with an average time to complication in cases of primary tumors of 24 months (range, 11–50 months) and 6 months (range, 1–9 months) in cases of metastatic disease. Two patients died of an acute myocardial infarction in the early postoperative period and were excluded from the functional evaluation.
The implant is FDA-approved for use in the femur, tibia, and humerus (Fig. 1A–C). The design consists of a central spacer clamped onto a proximal and distal intramedullary nail (Fig. 2) with a grit-blasted coating that is designed to accept appositional bone. The spacers are clamped around the proximal and distal intramedullary nails, which are inserted into native bone with or without PMMA and secured together using screws and a torque-limiting screwdriver. In noncemented implants, proximal and distal transfixion screws were used for fixation of the intramedullary nails. One surgeon used noncemented fixation, whereas all other surgeons used cemented fixation. Tumors were treated using standard oncologic principles. Primary tumors were treated with wide margins, which resulted in large defects, and the intraoperative pathology consult confirmed negative margins. Canals were reamed with flexible power reamers proximally and distally and cemented or noncemented fixation was used as described previously. Endoprosthetic body segments were placed and proper rotation was determined by preresection marks placed in the proximal and distal aspects of the affected bone. Once the endoprosthesis was in place, soft tissue coverage was performed using additional flap coverage if deemed necessary by the surgeon. Intraoperatively the integrity of the prosthesis was imaged by fluoroscopic imaging. All perioperative events were recorded regarding complications and functional evaluations were performed using the MSTS-International Society of Limb Salvage criteria [14] when rehabilitation was complete or at latest followup if rehabilitation was unable to be completed. Patients were followed clinically and with radiographic imaging at 1-month intervals for 3 months followed by 3-month intervals for the remainder of the first 1 year after time of surgery with subsequent followup every 6 months. Each surgeon classified complications according to the five modes of failure for prosthetics proposed by Henderson et al. [19, 27] and all classifications were reviewed by two surgeons (RK, MJF) and the senior author (JB). Henderson failure Types 1 to 4 were subsequently grouped as nononcologic failures for further analysis.
All patient analysis was conducted with regard to survivorship, complications, site of complication, functional outcomes, and fixation method. Statistical outcomes were measured using the chi-square test and t-test for two proportions with a level of significance set at p < 0.05. All statistical analyses were performed using GraphPad Prism Software (Version 6; GraphPad Software, Inc, La Jolla, CA, USA).
Results
Overall, there were 12 implant-related complications (27%) with 11 of these being either Type II or Type III failures (Table 2). Of these 11, five cases (11%, Patients 7, 14, 15, 20, and 23) had Type II (aseptic loosening) failure, which occurred at the distal stem of the prosthesis at the bone-stem interface and six cases (14%, Patients 5, 6, 16, 26a, 40a, and 40b) had Type III (structural) failures, which occurred at the clamp-rod interface. Four of the five cases of Type II failure were associated with breakage of the distal locking screw (Patients 7, 14, 15, and 20). Of the six cases of Type III failure, three patients had failure of the reducing bushing within the prosthesis (Patients 5, 26a, and 40a) and were revised with a new spacer clamp (Fig. 2). One patient (Patient 40b) experienced a second failure of the spacer clamp and was converted to an autograft-nail construct. Two other failures in this group occurred after a traumatic fall causing loosening of the clamp spacer and malrotation in one (Patient 6), which was corrected through revision surgery, and breakage of a connecting spacer in the other (Patient 16) requiring conversion to a total femoral replacement. One patient who previously experienced a Type III failure and was revised with a new clamp subsequently experienced a Type IV failure (infection), which required removal of the implant and conversion to a total femur replacement (Patient 26b). There was one case of local recurrence in a patient with metastatic renal cell carcinoma who required an above-knee amputation as a result of disease progression (Patient 37). We classified this patient as having a Type V (local recurrence) failure.
All 12 nononcologic complications occurred in patients with femoral implants. When compared with other anatomic sites, complications were more common in femoral sites (12 of 21 procedures [57%]) than in either tibial or humeral sites (0 of 23 procedures; odds ratio [OR], 62; 95% confidence interval [CI], 3–1154; p < 0.0001). Complication rate was not associated with resection size or intramedullary stem dimensions.
Overall, patients in this series had a mean MSTS score of 77% (humerus = 83%, tibia = 77%, femur = 75%) and additional detail for MSTS scoring can be seen in Table 1. Patients with cemented implants had higher MSTS scores than those with noncemented fixation (84% versus 66%, p = 0.0017). To assess the potential role of increased followup and implant age on functional scores, MSTS scores and subscores were grouped by anatomic site and then by duration of followup into 2 groups: (1) cases with less than 1 year followup and (2) cases with 1 year of followup or more. Duration of followup was not associated with MSTS scores for any anatomic sites. Resection size and intramedullary dimensions were not associated with either overall MSTS scores or subscores as assessed by the MSTS system.
The complication rate was 33% in noncemented cases and 21% in cemented cases (p = 0.39). In cases of noncemented fixation, all five nononcologic failures were Type II failures and occurred at the bone-stem interface. In cases of cemented fixation, all six nononcologic failures were Type III failures and occurred at the clamp-rod implant interface. Noncemented fixation of femoral reconstructions was associated with Type II failure and cemented fixation in the femur was associated with Type III failure (OR, 143; 95% CI, 2.413–8476; p = 0.0022).
Discussion
Reconstruction of segmental skeletal defects in long bones after oncologic resection presents a challenge for orthopaedic surgeons. There are several methods of reconstruction for these defects, which include autografts, allografts, and endoprostheses. The goals of surgery are to relieve pain, achieve tumor control, and preserve function. Ideally, the reconstruction would provide immediate stability, preservation and early motion of adjacent joints and survival for the lifespan of the patient [10, 13, 35]. This study presents the functional outcomes of 44 reconstructive operations in 41 patients using a modular intercalary endoprosthesis. These results are comparable to similar studies involving intercalary endoprostheses and the inclusion of 41 patients (44 implants) from three musculoskeletal oncology centers makes this one of the largest studies to date using this type of reconstruction [1, 2, 9, 10, 18, 24, 31, 33] (Table 3).
This study has several limitations which bear discussion. First, this is a retrospective study and as such is subject to recall and selection bias. Notably, this may have contributed to our inability to find a difference in complication rate based on the use of cemented PMMA versus press-fit stems for fixation. Second, this study lacks a true control group because all cases included in this study fall under a single clinical indication and only one treatment was used for this indication; thus, we cannot directly compare our results with other types of implants, biologic reconstructions, or other more conservative treatments for other indications such as radiation alone or radiation combined with internal fixation. Third, the types of tumors included in this study as well as our patient population includes both cases of primary bony tumors and metastatic lesions in patients with notably different life expectancies as a result of variation in age and tumor stage at the time of treatment. Fourth, three (7%) of our patients were lost to followup and we cannot account for the lifespan of these implants in our final analysis.
We found a 27% overall nononcologic complication rate (12 of 44 procedures experienced complications) in our patients treated with this intramedullary endoprosthesis, which is similar when compared with the other options currently available. Published reports in the literature have complication rates ranging from 14% to 50% with mechanical failure of the prosthesis and aseptic loosening of the proximal or distal stem as the most common complications [1, 9]. Intercalary allografts allow attachment of soft tissues and come in various sizes, but patients are subject to a longer period of postoperative immobilization to protect the reconstruction until graft union [20]. They are also associated with complications, including nonunion (18%–64%) [12, 15, 22, 23], fracture of the allograft (15%–51%) [5, 12, 23, 25], and a risk of infection of up to 30% [12, 15, 26]. In these studies of intercalary allografts, most patients were treated for primary bone sarcomas and are not directly comparable to the majority of patients in this study. Vascularized autografts are another option and may heal and hypertrophy under mechanical loads, but it may take several years before the graft will allow full weightbearing [17]. Additionally, these grafts may have size limitations and are associated with donor site morbidity [7, 29]. Extracorporeally irradiated autografts can be used as an alternative to allografts to reconstruct a defect but require a longer time to incorporate and are therefore subject to nonunion, fracture, and infection [8]. In addition, the potential loss of structural integrity of the resected bone segment limits the use of this technique. Distraction osteogenesis and bone transport may provide adequate biomechanical strength but may be time-consuming (1 mm/day) and has the drawbacks of external fixation with pin tract problems, which may pose an increased infection risk, especially in patients receiving adjuvant radiotherapy and chemotherapy [13, 36]. Reconstruction with an endoprosthesis avoids the prolonged period of immobilization associated with auto- and allograft reconstructions and allows early weightbearing. Early endoprostheses were limited by the lack of modularity. Lap joint endoprostheses are capable of using various stem-body lengths to create the prosthesis, but the segments are a one-piece design and sizes are more limited [9, 10]. Custom-made prostheses require several weeks to fabricate. In contrast, the IDSF system provides a modular design allowing the surgeon to intraoperatively adjust the implant to the proper size with various stem and spacer dimensions to fill the defect and correct any limb length discrepancy [31]. A recent biomechanical study has demonstrated systematically equivalent or greater load resistance of modular segmental endoprostheses in all types of loading (axial compression, four-point bending, internal and external torsion) when compared with other fixation techniques [32].
Complications in our study were limited to patients with femoral implants. Although the overall incidence of complications in this study is 27%, which is similar to other studies [1, 2, 4, 9, 10, 18, 24, 31, 33] (Table 3), 57% of femoral reconstructions developed complications in this series.
We observed that the average functional results of all patients as measured by MSTS scores was 77% and this is comparable to that seen in other studies of intercalary endoprosthesis [9, 18, 24, 31, 33]. This approach seemed to provide adequate pain relief and maintenance of function, most notably with respect to the reduced postoperative time until full weightbearing could be permitted, in a group of patients who have a relatively short expected lifespan.
The use of PMMA for fixation of this intercalary endoprosthesis may be advantageous because our results and other studies have demonstrated increased postoperative function as measured by MSTS scores in patients with cemented implants [16, 21]. Failure of this implant remains a concern in cases of femoral reconstruction and this study demonstrates that cemented reconstructions are associated with failure at the clamp-rod interface (Type III), whereas noncemented fixation is associated with aseptic loosening and stem failure (Type II). Cemented fixation may also be beneficial because revision of stem (Type II) failure is surgically more challenging than revision at the clamp-rod interface.
The surgeon and patient need to consider the various options of treatment and especially whether to goal is palliation versus an attempt to increase disease control in patients with metastatic carcinoma. This prosthesis has value in patients with a solitary bony metastasis in a long bone with shortened expected longevity. Various autografts, allografts, and prostheses have all been proposed, each with its own advantages and disadvantages. The results of this study and those of similar studies indicate that the IDSF endoprosthesis can be used with results that seem similar to those of reported with other reconstructions. Because there has been an emphasis on early weightbearing and return to normal activity in patients with limited life expectancy, immediate stability provided by an intercalary endoprosthesis makes this a reasonable alternative to consider. In conclusion, the authors recommend caution in its use at the femoral site as a result of its high rate of complication. For cases of solitary tumors in the humerus and tibia, which cannot be otherwise reconstructed using biologic options, we recommend the use of the IDSF system with PMMA for fixation.
References
Abudu A, Carter SR, Grimer RJ. The outcome and functional results of diaphyseal endoprostheses after tumour excision. J Bone Joint Surg Br. 1996;78:652–657.
Ahlmann ER, Menendez LR. Intercalary endoprosthetic reconstruction for diaphyseal bone tumours. J Bone Joint Surg Br. 2006;88:1487–1491.
Aksnes LH, Bauer HC, Jebsen NL, Folleras G, Allert C, Haugen GS, Hall KS. Limb-sparing surgery preserves more function than amputation: a Scandinavian sarcoma group study of 118 patients. J Bone Joint Surg Br. 2008;90:786–794.
Aldlyami E, Abudu A, Grimer RJ, Carter SR, Tillman RM. Endoprosthetic replacement of diaphyseal bone defects. Long-term results. Int Orthop. 2005;29:25–29.
Alman BA, De Bari A, Krajbich JI. Massive allografts in the treatment of osteosarcoma and Ewing sarcoma in children and adolescents. J Bone Joint Surg Am. 1995;77:54–64.
Brien EW, Terek RM, Healey JH, Lane JM. Allograft reconstruction after proximal tibial resection for bone tumors. An analysis of function and outcome comparing allograft and prosthetic reconstructions. Clin Orthop Relat Res. 1994;303:116–127.
Chang DW, Weber KL. Use of a vascularized fibula bone flap and intercalary allograft for diaphyseal reconstruction after resection of primary extremity bone sarcomas. Plast Reconstr Surg. 2005;116:1918–1925.
Chen TH, Chen WM, Huang CK. Reconstruction after intercalary resection of malignant bone tumours: comparison between segmental allograft and extracorporeally-irradiated autograft. J Bone Joint Surg Br. 2005;87:704–709.
Damron TA, Leerapun T, Hugate RR, Shives TC, Sim FH. Does the second-generation intercalary humeral spacer improve on the first? Clin Orthop Relat Res. 2008;466:1309–1317.
Damron TA, Sim FH, Shives TC, An KN, Rock MG, Pritchard DJ. Intercalary spacers in the treatment of segmentally destructive diaphyseal humeral lesions in disseminated malignancies. Clin Orthop Relat Res. 1996;324:233–243.
Deijkers RL, Bloem RM, Kroon HM, Van Lent JB, Brand R, Taminiau AH. Epidiaphyseal versus other intercalary allografts for tumors of the lower limb. Clin Orthop Relat Res. 2005;439:151–160.
Donati D, Di Liddo M, Zavatta M, Manfrini M, Bacci G, Picci P, Capanna R, Mercuri M. Massive bone allograft reconstruction in high-grade osteosarcoma. Clin Orthop Relat Res. 2000;377:186–194.
Dormans JP, Ofluoglu O, Erol B, Moroz L, Davidson RS. Case report: Reconstruction of an intercalary defect with bone transport after resection of Ewing’s sarcoma. Clin Orthop Relat Res. 2005;434:258–264.
Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246.
Gebhardt MC, Flugstad DI, Springfield DS, Mankin HJ. The use of bone allografts for limb salvage in high-grade extremity osteosarcoma. Clin Orthop Relat Res. 1991;270:181–196.
Habermann ET, Sachs R, Stern RE, Hirsh DM, Anderson WJ Jr. The pathology and treatment of metastatic disease of the femur. Clin Orthop Relat Res. 1982;169:70–82.
Han CS, Wood MB, Bishop AT, Cooney WP 3rd. Vascularized bone transfer. J Bone Joint Surg Am. 1992;74:1441–1449.
Hanna SA, Sewell MD, Aston WJ, Pollock RC, Skinner JA, Cannon SR, Briggs TW. Femoral diaphyseal endoprosthetic reconstruction after segmental resection of primary bone tumours. J Bone Joint Surg Br. 2010;92:867–874.
Henderson ER, Groundland JS, Pala E, Dennis JA, Wooten R, Cheong D, Windhager R, Kotz RI, Mercuri M, Funovics PT, Hornicek FJ, Temple HT, Ruggieri P, Letson GD. Failure mode classification for tumor endoprostheses: retrospective review of five institutions and a literature review. J Bone Joint Surg Am. 2011;93:418–429.
Hornicek FJ, Gebhardt MC, Tomford WW, Sorger JI, Zavatta M, Menzner JP, Mankin HJ. Factors affecting nonunion of the allograft-host junction. Clin Orthop Relat Res. 2001;382:87–98.
Jacofsky DJ, Haidukewych GJ. Management of pathologic fractures of the proximal femur: state of the art. J Orthop Trauma. 2004;18:459–469.
Makley JT. The use of allografts to reconstruct intercalary defects of long bones. Clin Orthop Relat Res. 1985;197:58–75.
Mankin HJ, Gebhardt MC, Jennings LC, Springfield DS, Tomford WW. Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res. 1996;324:86–97.
McGrath A, Sewell MD, Hanna SA, Pollock RC, Skinner JA, Cannon SR, Briggs TW. Custom endoprosthetic reconstruction for malignant bone disease in the humeral diaphysis. Acta Orthop Belg. 2011;77:171–179.
Muscolo DL, Ayerza MA, Aponte-Tinao L, Ranalletta M, Abalo E. Intercalary femur and tibia segmental allografts provide an acceptable alternative in reconstructing tumor resections. Clin Orthop Relat Res. 2004;426:97–102.
Ortiz-Cruz E, Gebhardt MC, Jennings LC, Springfield DS, Mankin HJ. The results of transplantation of intercalary allografts after resection of tumors. A long-term follow-up study. J Bone Joint Surg Am. 1997;79:97–106.
Palumbo BT, Henderson ER, Groundland JS, Cheong D, Pala E, Letson GD, Ruggieri P. Advances in segmental endoprosthetic reconstruction for extremity tumors: a review of contemporary designs and techniques. Cancer Control. 2011;18:160–170.
Refaat Y, Gunnoe J, Hornicek FJ, Mankin HJ. Comparison of quality of life after amputation or limb salvage. Clin Orthop Relat Res. 2002;397:298–305.
Rose PS, Shin AY, Bishop AT, Moran SL, Sim FH. Vascularized free fibula transfer for oncologic reconstruction of the humerus. Clin Orthop Relat Res. 2005;438:80–84.
Rougraff BT, Simon MA, Kneisl JS, Greenberg DB, Mankin HJ. Limb salvage compared with amputation for osteosarcoma of the distal end of the femur. A long-term oncological, functional, and quality-of-life study. J Bone Joint Surg Am. 1994;76:649–656.
Ruggieri P, Mavrogenis AF, Bianchi G, Sakellariou VI, Mercuri M, Papagelopoulos PJ. Outcome of the intramedullary diaphyseal segmental defect fixation system for bone tumors. J Surg Oncol. 2011;104:83–90.
Sakellariou VI, Mavrogenis AF, Babis GC, Soucacos PN, Magnissalis EA, Papagelopoulos PJ. Comparison of four reconstructive methods for diaphyseal defects of the humerus after tumor resection. J Appl Biomech. 2012;28:568–578.
Sewell MD, Hanna SA, McGrath A, Aston WJ, Blunn GW, Pollock RC, Skinner JA, Cannon SR, Briggs TW. Intercalary diaphyseal endoprosthetic reconstruction for malignant tibial bone tumours. J Bone Joint Surg Br. 2011;93:1111–1117.
Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 1986;68:1331–1337.
Simon MA, Springfield DS. Surgery for Bone and Soft-tissue Tumors. Philadelphia, PA, USA: Lippincott-Raven Publishers; 1998.
Tsuchiya H, Tomita K, Minematsu K, Mori Y, Asada N, Kitano S. Limb salvage using distraction osteogenesis. A classification of the technique. J Bone Joint Surg Br. 1997;79:403–411.
Author information
Authors and Affiliations
Corresponding author
Additional information
One or more of the authors (JB) has received funding from the Musculoskeletal Transplant Foundation and has also received fees from Merete Inc (Berlin, Germany) and Implant Cast (Buxtehude, Germany) outside of the submitted work and holds several patents and licensing fees with CreOsso LLC (Montclair, NJ, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Rutgers New Jersey Medical School, Newark, NJ, USA; the University Clinic of Bonn, Bonn, Germany; and University Medical Center Schleswig-Holstein, Campus Lübeck, Lübeck, Germany.
About this article
Cite this article
Benevenia, J., Kirchner, R., Patterson, F. et al. Outcomes of a Modular Intercalary Endoprosthesis as Treatment for Segmental Defects of the Femur, Tibia, and Humerus. Clin Orthop Relat Res 474, 539–548 (2016). https://doi.org/10.1007/s11999-015-4588-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-015-4588-z