Abstract
Background
Undiagnosed low-grade prosthetic joint infections (PJI) are recognized as an important reason for early failure of presumably aseptic revisions. Preoperatively administered antimicrobial prophylaxis reduces the incidence of PJI but it may reduce the sensitivity of microbiologic periprosthetic tissue cultures and consequently increase the incidence of undiagnosed septic prosthetic joint failures, which can lead to catastrophic serial revisions.
Questions/purposes
We wished to determine whether administration of preoperative antibiotics decreases the likelihood of diagnosing PJI in patients undergoing revision hip or knee arthroplasty in whom infection is suspected.
Methods
We prospectively enrolled and evaluated 40 patients (29 with THAs and 11 with TKAs) who met the following inclusion criteria: older than 18 years, with suspected PJI of unknown cause, undergoing surgical revision. After arthrotomy, three tissue samples were obtained for microbiologic analysis and diagnosis, and antimicrobial prophylaxis (cefazolin 2 g intravenously) then was administered. Later during the procedure, but before débridement and irrigation, the second set of three tissue samples was obtained from the same surgical area and was cultured. Tissue concentration of prophylactic antibiotic was verified with the second set of samples. A positive culture result was defined as one or more positive cultures (growth on agar at or before 14 days). We then compared the yield on the microbiologic cultures obtained before administration of antibiotics with the yield on the cultures obtained after antibiotics were administered. An a priori analysis was performed; with the numbers available, we had 98% power to detect a difference in diagnostic sensitivity of 33%.
Results
With the numbers available, we found no difference in the likelihood that an infection would be diagnosed between the samples obtained before and after administration of antimicrobial prophylaxis (odds ratio [OR] for positive microbial culture = 0.99; 95% CI, 0.40–2.48; p = 0.99). All measured tissue concentrations of cefazolin were greater than the minimum inhibitory concentration, therefore we found that antibiotic prophylaxis was adequate at the time of second-set tissue specimen recovery.
Conclusions
Results from this small, prospective series suggest that preoperative antimicrobial prophylaxis may be administered safely even in patients undergoing revision hip or knee arthroplasty in which microbiologic sampling is planned without compromising the diagnostic sensitivity of tissue sample cultures. However, before applying our results more generally, our findings need to be confirmed in larger, multicenter studies that would allow evaluation by sex, procedure, bacteriology, and other potentially important factors.
Level of Evidence
Level I, diagnostic study.
Similar content being viewed by others
Introduction
Although prosthetic joint infections (PJI) are relatively uncommon, because the numbers of primary and revision total joint arthroplasties are high and increasing, PJIs will impose a large and growing financial burden [6, 10, 25, 26]. It is increasingly recognized that many presumably aseptic revisions are infected [2, 16]. Because treatment of PJI differs considerably from the treatment of aseptic failure, it is important to distinguish the two entities before treatment [26]. The diagnosis of PJI can be challenging, particularly in patients with low-grade infections with nonspecific clinical presentation and the absence of systemic laboratory markers of inflammation. An inappropriate assumption that a potentially septic process is actually an aseptic one leads to repeat revisions with serial early failures and gradual function loss, the mental and physical costs, time loss, and sometimes limb loss or death [16].
Identification of the causative agent of infection is crucial for successful treatment of PJIs. Patients with painful arthroplasties and patients with premature loosening should undergo screening for PJI before surgery [22]. The more accurate the diagnostic evaluation is before revision, the greater the success rate. A thorough evaluation should include a complete history, physical examination, radiographs, measurement of serum levels of C-reactive protein, synovial fluid aspiration with cell count and differential, and use of aerobic and anaerobic cultures [1, 3, 18]. Despite clinicians’ efforts, the diagnosis of a low-grade infection sometimes may be missed before revision surgery. Tissue samples obtained intraoperatively are the “gold standard” for diagnosing a PJI [3], although the sensitivity ranges between 60% and 70% depending on different factors [1], and they are subject to false-positive and false-negative results.
Preoperative antibiotic prophylaxis decreases the risk of postoperative infections and therefore has become standard care in joint replacement surgery [9, 11]. However, in revision total joint arthroplasties, there is disagreement regarding whether preoperative antibiotic prophylaxis given just before surgery may cause false-negative intraoperative tissue culture results [4, 17, 19, 20, 23]. Because of this, some authors have proposed deferring preoperative antibiotic prophylaxis until after tissue samples have been collected for culture [19, 20, 23], whereas others have claimed that preoperative antibiotic prophylaxis does not affect intraoperative tissue cultures [4, 17].
We therefore wanted to analyze the influence of preoperative antibiotic prophylaxis on results of intraoperative tissue cultures in patients undergoing revision hip or knee arthroplasty and in whom infection is suspected. We used the same patients for the study and control groups by taking samples from the same surgical sites before and after administration of antibiotics to avoid sampling bias. Our null hypothesis was that preoperative antibiotic prophylaxis does not influence the sensitivity of diagnosing PJI using periprosthetic tissue cultures.
Patients and Methods
This study received approval from the Republic of Slovenia National Medical Ethics Committee. Between 2012 and 2013, we treated 51 patients for suspected PJI with a THA or TKA. We invited patients to participate in this prospective study who met the following inclusion criteria: early loosening (< 10 years after primary implantation), positive synovial cytology results and negative microbiology results on preoperative aspiration (in this series, all aspirations performed yielded sufficient fluid on which to perform cultures), and any TKA or THA with no obvious mechanical reason for failure (migration of prosthesis) [24]. We did not invite patients who met these exclusion criteria: antimicrobial therapy less than 14 days before surgery, tourniquet was used during surgery, violation of study protocol, a prosthetic heart valve present, or allergy to cephalosporin. Mechanical and extraarticular causes for failure were diagnosed by clinical examination and imaging studies [22]. This resulted in 40 (78%) patients undergoing surgery for PJI who were eligible for participation in the study. Of those, all consented to participate, and these patients represented our study group; this group included 29 patients undergoing surgery for infected THAs and 11 for infected TKAs. All patients underwent single-stage revision surgery. The same patients comprised the study and control groups in that we took cultures before and after antibiotic administration in the same patients. This was a single-institution study (Orthopedic Hospital Valdoltra). Surgical revision was performed and the infecting organism was unknown before the surgery.
The mean age of the study population was 75.8 years (± 8.9 years), with the range from 50 to 87 years. Twenty of the 40 patients were men.
Patients were prepared for surgery in a standard manner depending on localization of the surgical site. Tourniquets were not used during the revision TKAs. After reaching the infected site, three periprosthetic soft tissue specimens, approximately 0.5 cm3 each, were obtained for microbiologic testing. Areas with the most visually inflamed tissue were sampled. After obtaining the first set of three tissue specimens, antimicrobial prophylaxis was administered (2 g cefazolin intravenously). The surgical procedure was performed in a standard manner but débridement of the site of original tissue sampling was deferred until after harvesting the second set of three cultures. Finally, the second set of three tissue specimens of approximately the same size and from the same surgical area was obtained. At the same time a tissue sample from the same area was collected for verification of the concentration of prophylactic antibiotic. Tissue specimens for microbiologic testing were placed in separate sterile containers, labeled with the patient’s name and number, transported to the microbiology laboratory, and processed within 24 hours.
The microbiologist (MK) was blinded to which tissue specimens were obtained before and which after antimicrobial prophylaxis was initiated. In the microbiology laboratory, each container with a tissue specimen was weighed on an analytical scale (Type AE 200; Mettler-Toledo International, Columbus, OH, USA). Each tissue specimen was transferred to 5 mL of sterile normal saline (0.9% NaCl) and homogenized using a Masticator Silver/Panoramic digital blender (IUL Instruments GmbH, Konigswinter, Germany) for 90 seconds (5 strokes/second). The homogenate was serially diluted twice to a 1:10 ratio by transferring 0.5 mL of the homogenate into 4.5 mL of sterile normal saline. Aliquots of 0.1 mL of undiluted homogenate and each dilution were plated on agar plates in aerobic conditions (Columbia blood agar and chocolate agar Anaerobe Systems, Morgan Hill, CA, USA) and anaerobic conditions (Brucella blood agar, Anaerobe Systems). Each empty container was weighed and its weight subtracted from the filled container weight to calculate the weight of each tissue specimen. Columbia blood agar plates and enrichment thioglycollate broths (Anaerobe Systems) were incubated at 35° C in ambient air, chocolate agar plates at 35° C with 5% CO2, and Brucella blood agar plates at 35° C in an anaerobic atmosphere. All agar plates were examined daily for 14 days. The growth of any microorganisms on the plates was recorded and colony-forming units (CFU) with the same morphologic features were enumerated and expressed per gram of tissue (considering the dilution factor). Microorganisms were identified using standard identification methods and their antimicrobial susceptibility was determined using disc-diffusion testing according to the Clinical and Laboratory Standards Institute guidelines [5]. All isolates of different species were stored at –70° C in MicrobankTM vials (Pro-Lab Diagnostics UK, Merseyside, UK).
Tissue concentrations of prophylactic antibiotic were verified using the tissue sample harvested just before the second set of tissue samples was taken. Tissue samples were extracted with 2 mL of 0.1 mol Sorensen’s phosphate buffer, centrifuged, and supernatant was applied on the solid phase extraction column (Phenomenex LTD, Aschaffenburg, Germany). Plasma samples were diluted with water (1:3 plasma volume to water volume ratio) before solid phase extraction. The solutions were applied on activated StrataTM-X 33-μm polymeric sorbent, 100 mg/6 mL columns (Phenomenex LTD) and cefazolin was eluted with 3 mL methanol. Samples were dried and residues dissolved in 1 mL of mobile phase, prepared by mixing 770 mL of 0.02 mol phosphate buffer (pH 5) with 250 mL of methanol. Fifty microliters of sample solution then was injected in the Agilent 1260 diode array detector (Agilent Technology, Palo Alto, CA, USA) with a Gemini® 5 µm C18 110A chromatographic column (Phenomenex) used for the separation. Cefazolin stock solution with a concentration of 1.0 mg/mL was prepared by dissolving cefazolin sodium salt (Sigma Aldrich, St Louis, MO, USA) in the mobile phase. Extracted matrix-matched calibration curves, ranging from 5 mg to 100 mg cefazolin/kg, were used to assess the cefazolin concentration in samples.
The purpose of measuring tissue antibiotic concentration at the time of the second set of tissue specimen recovery was to establish a control point. We wanted to show that if there was a difference between the samples, antibiotic prophylaxis would be adequate at the time of recovery of the control tissue specimens. Staphylococcus species should be considered susceptible if the minimum inhibitory concentration (MIC) for cefazolin is 2 μg/mL or less [7, 8]. In our study, all measured tissue concentrations of cefazolin were greater than the MIC, therefore we found that antibiotic prophylaxis was adequate at the time of second-set tissue specimen recovery.
Statistical Analysis
Power analysis of our study sample achieved 98% power to detect a difference of 0.330 (a 33% difference in diagnostic sensitivity) between the area under the receiver operating characteristic (ROC) curve, the area under the curve (AUC), with the null hypothesis of 0.5000 and an AUC for the alternative hypothesis of 0.830 using a two-sided z test at a significance level of 0.050. The data were discrete (rating scale) responses. The AUC was computed between false-positive rates of 0.00 and 1.00. The ratio of the SD of the results in the negative group to those in the positive group was 1.00. The AUC is the probability that the predicted value of the sample with the outcome variable equal to one will be ranked higher than the predictive value for the sample with the outcome variable equal to 0. Continuous data were presented as mean ± SD or median with interquartile range. Categoric data were expressed as frequencies or percentages. As the number of CFU per gram of tissue was an asymmetrically distributed variable, the nonparametric Wilcoxon signed rank test was used to estimate the difference in the outcome measure between pre- and postantimicrobial prophylaxis-obtained tissue samples. The possible effect of a tissue specimen as a random factor and timing of tissue specimen recovery (before/after antimicrobial prophylaxis) as a fixed factor, on the number of CFU per gram of tissue were tested using ANOVA with repeated measures. A logistic-regression model was used to assess the relationship between the timing of tissue specimen recovery and qualitative assessment of microbial cultures. Three measurements for each statistical unit, for measurement before and after separately, were combined into one new variable—the assessment of culture positivity. For the purpose of analysis, patients with all three tissue specimens found to be negative were defined as “negative”, whereas all others were defined as “positive”. Again, the tissue specimen as a random factor was included in the model. Results were presented as the odds ratio (OR) for positive microbial culture with 95% CI. A logistic regression model was used. The agreement in qualitative assessment of culture positivity between pre- and postantimicrobial prophylaxis-obtained tissue specimens was estimated by calculating the intraclass correlation coefficient (ICC). To estimate the predictive accuracy of postantimicrobial prophylaxis tissue microbial cultures for implant infection, the ROC approach was applied. For this purpose, the variable assessment of culture positivity was dichotomized. We took into account the negative and positive results obtained before antimicrobial prophylaxis and as a test method, and that we obtained after antimicrobial prophylaxis. Quality of discrimination was assessed by calculating the area under the ROC curve (the AUC), the sensitivity, specificity, positive predictive value, and negative predictive value. All estimates were reported with 95% CIs.
A p value less than 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS® Version 20.0 software (IBM Corporation, Armonk, NY, USA).
Results
We found no differences with the numbers available between tissue samples taken before and after administration of antimicrobial prophylaxis (OR, 0.99; 95% CI, 0.40–2.48). Comparison of numbers of CFU per gram of tissue specimens before and after prophylaxis did not show a statistical difference: the mean number of CFU per gram of tissue, averaged across all three specimens, was not different if the specimens were obtained before or after antimicrobial prophylaxis (Wilcoxon signed rank test, p = 0.280). Almost identical results were obtained if the medians instead of the means were calculated for the three tissue specimens (median before prophylaxis, 3438; SD, 13,726; 95% CI, −816 to 7691; median after prophylaxis, 18,233; SD, 105,415; 95% CI, −14,434 to 50,901; p = 0.25).
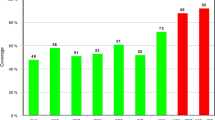
To retain the information of each tissue specimen culture result, two-way ANOVA with repeated measures was conducted. Neither the time of specimen recovery (before or after the antimicrobial prophylaxis) nor the tissue specimen per se proved to be statistically significant in relation to number of CFUs per gram of tissue (F = 0.22; p = 0.64, and F distribution = 0.80; p = 0.46, respectively). We found the incidence of a different number of negative or positive tissue specimens obtained before and after antimicrobial prophylaxis (Fig. 1).
The distribution of positive and negative periprothetic microbial cultures in three tissue specimens for the 40 patients, before and after antimicrobial prophylaxis, is shown. NNN = all three tissue specimens were negative; PNN = one tissue specimen was positive and two were negative; PPN = two tissue specimens were positive and one was negative; PPP = all three tissue specimens were positive.
Using the ROC curve approach, we confirmed that qualitative assessment of culture positivity on the basis of samples taken after antimicrobial prophylaxis compared with samples taken before antimicrobial prophylaxis was an accurate method (AUC, 0.83; 95% CI, 0.70–0.97) (Fig. 2). Sensitivity was 79% (95% CI, 58%–93%), specificity was 88% (95% CI, 62%–98%), positive predictive value was 91% (95% CI, 70%–99%), and negative predictive value was 74% (95% CI, 49%–91%). The ICC showed good agreement in qualitative assessment of microbial tissue cultures between samples taken before and after antimicrobial prophylaxis (ICC = 0.78; 95% CI, 0.63–0.88; p < 0.001).
In our study, all measured tissue concentrations of cefazolin were higher than the MIC, therefore we found that antibiotic prophylaxis was adequate at the time of second-set tissue specimen recovery.
A total of 85% (49/58) of the detected microorganisms were susceptible to the antibiotic prophylaxis that was administered (Table 1).
Discussion
Antimicrobial prophylaxis is used routinely before surgery to reduce patients’ risk of infection with a primary arthroplasty [9]. It is common to defer antimicrobial prophylaxis until after tissue samples have been obtained in revision arthroplasties with unidentified organisms before surgery. The potential advantage is to identify an organism not identified through preoperative aspiration. The disadvantage is that patients are deprived of the benefit of antimicrobial prophylaxis before skin incision, a benefit that has been called the greatest single contribution to minimizing infection in total joint arthroplasties [13, 14], especially in revisions where infection rates can range from 5% to 10%, and has been documented as much as 40% in different studies [12, 15, 21]. This risk discrepancy is likely multifactorial, but withholding antimicrobial prophylaxis until after cultures are obtained intraoperatively may contribute to the higher rates of PJIs observed in patients undergoing revision arthroplasties. A true PJI frequently is treated as aseptic loosening. If it is not recognized preoperatively, the condition leads to early failures and repeat revision procedures. We found no difference in diagnostic yield between cultures taken before and after administration of parenteral antibiotics in patients in whom PJI was suspected.
The main limitation of our study is the relatively small sample size and because of that, there is no evaluation by procedure, patient sex, or bacteriology. Another limitation is that the “gold standard” for this diagnosis is unknown (tissue cultures are one standard and 2 years clinical followup is another). We did not follow these patients long enough to see if negative cultures (in either group) were true negatives, defined as the absence of clinical infection after a sufficient period of observation. In addition, these results might not apply to a population in whom PJI was not suspected, as the bacterial burden might be different. In addition, in cases of difficult-to-detect bacteria, all microorganisms might be eliminated with prophylaxis, and in cases of high bacterial load prophylaxis might not matter. Finally, in terms of statistical power, with the numbers available, we had 98% power to detect a difference in diagnostic sensitivity of 33%.
Two prior studies found, as we did, that preoperative antibiotics do not decrease the diagnostic yield of intraoperative cultures [4, 17]. Our results confirm and extend those findings, as our study was designed to address some of the methodologic shortcomings in those reports. It is essential that patients assigned to treatment and control groups be representative of the same population. Our control group was the same population, not just a similar population. We studied a general population with suspected PJI in whom the infecting organism was unknown before the surgery. It is the first study, to our knowledge, addressing this question. Although tissue antibiotic concentration was greater than the MIC at the time control samples were collected, there was still no difference in sensitivity with the numbers available between the samples obtained before and after administration of antimicrobial prophylaxis. However even with three negative samples, infection with difficult to detect bacteria might have been missed in both groups. Sonication and PCR might be better methods to confirm PJI, however we did not use either method in our study.
Some authors have proposed deferring preoperative antibiotic prophylaxis until after tissue samples have been collected for culture [19, 20, 23], because they fear not identifying the causative agent of the infection, which is the most important element in the treatment of PJI. There are no prospective studies of which we are aware that show that this practice is justified.
Results from our small, prospective series support those of other reports [4, 17], and suggest that a single dose of antimicrobial prophylaxis does not alter intraoperative culture results in PJI. There were no differences with the numbers available in the concordance rate between preoperative and intraoperative cultures. Results from this prospective series of patients with potential PJIs suggest that antimicrobial prophylaxis will not substantially compromise the diagnostic sensitivity of tissue sample cultures in revision hip and knee arthroplasties even if administered before microbiologic sampling. However, before applying these results to all revisions, our findings need to be confirmed in a larger, multicenter population that would allow evaluation by sex, procedure, bacteriology and other potentially important factors.
References
Berbari E, Mabry T, Tsaras G, Spangehl M, Erwin PJ, Murad MH, Steckelberg J, Osmon D. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2010;92:2102–2109.
Bereza PL, Ekiel A, Auguściak-Duma A, Aptekorz M, Wilk I, Kusz DJ, Wojciechowski P, Martirosian G. Identification of silent prosthetic joint infection: preliminary report of a prospective controlled study. Int Orthop. 2013;37:2037–2043.
Bjerkan G, Witsø E, Nor A, Viset T, Løseth K, Lydersen S, Persen L, Bergh K. A comprehensive microbiological evaluation of fifty-four patients undergoing revision surgery due to prosthetic joint loosening. J Med Microbiol. 2012;61:572–581.
Burnett RS, Aggarwal A, Givens SA, McClure JT, Morgan PM, Barrack RL. Prophylactic antibiotics do not affect cultures in the treatment of an infected TKA. Clin Orthop Relat Res. 2010;468:127–134.
Clinical and Laboratory Standards Institute. [Please insert the proper title of the guidelines here]. Available at: http://clsi.org/standards/. Accessed July 7, 2015.
Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422–1429.
Dien Bard J, Hindler JA, Gold HS, Limbago B. Rationale for eliminating Staphylococcus breakpoints for β-lactam agents other than penicillin, oxacillin or cefoxitin, and ceftaroline. Clin Infect Dis. 2014;58:1287–1296.
EUCAST, European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 4.0. http://www.eucast.org. Accessed September 24, 2014.
Harrasser N, Harnoss T. [Prevention of periprosthetic joint infections] [in German]. Wien Med Wochenschr. 2012;162:115–120.
Jaekel DJ, Ong KL, Lau EC, Kurtz SM. The epidemiology of total joint arthroplasty infections. In: Trebše R, ed. Infected Total Joint Arthroplasty – the Algorithmic Approach. London: Springer-Verlag; 2012:35–54.
Jahoda D, Nyc O, Pokorný D, Landor I, Sosna A. [Antibiotic treatment for prevention of infectious complications in joint replacement][in Czech]. Acta Chir Orthop Traumatol Cech. 2006;73:108–114.
McElroy MJ, Pivec R, Issa K, Harwin SF, Mont MA. The effects of obesity and morbid obesity on outcomes in TKA. J Knee Surg. 2013;26:83–88.
Mulvey T, Thornhill T. Infected total knee arthroplasty. In: Insall JN, Scott WN, eds. Surgery of the Knee. 3rd ed. Philadelphia, PA, USA: Churchill Livingstone; 2001:1877–92.
Norden CW. Antibiotic prophylaxis in orthopaedic surgery. Rev Infect Dis 1991;13:842–6.
Peel TN, Buising KL, Choong PF. Prosthetic joint infection: challenges of diagnosis and treatment. ANZ J Surg. 2011;81:32–9.
Portillo ME, Salvadó M, Alier A, Sorli L, Martínez S, Horcajada JP, Puig L. Prosthesis failure within 2 years of implantation is highly predictive of infection. Clin Orthop Relat Res 2013;471:3672–8.
Tetreault MW, Wetters NG, Aggarwal V, Mont M, Parvizi J, Della Valle CJ. The Chitranjan Ranawat Award: Should Prophylactic Antibiotics Be Withheld Before Revision Surgery to Obtain Appropriate Cultures? Clin Orthop Relat Res 2014;472:52–6.
Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med 2004;117:556–62.
Trampuz A, Steckelberg JM, Osmon DR, Cockerill FR, Hanssen AD, Patel R. Advances in the laboratory diagnosis of prosthetic joint infection. Rev Med Microbiol 2003;14:1–14.
Trampuz A, Widmer AF. Infections associated with orthopedic implants. Curr Opin Infect Dis. 2006;19:349–56.
Trampuz A, Zimmerli W. Prosthetic joint infections: update in diagnosis and treatment. Swiss Med Wkly. 2005;135:243–251.
Trebše R. The diagnostic protocol for evaluation of periprosthetic joint infection. Hip Int 2012;22:25–35.
Trebse R, Pisot V, Trampuz A. Treatment of infected retained implants. J Bone Joint Surg Br 2005;87:249–56.
Trebše R, Trampuž A. Classification of Prosthetic Joint Infections. In: Trebše R, ed. Infected Total Joint Arthroplasty. London: Springer, 2012:31–34.
Vegari DN, Parvizi J. Joint arthroplasty and infection: where do we stand? J Long Term Eff Med Implants 2011;21:225–32.
Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med 2004;351:1645–54.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research ® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Valdoltra Orthopaedic Hospital, Ankaran, Slovenia.
About this article
Cite this article
Bedenčič, K., Kavčič, M., Faganeli, N. et al. Does Preoperative Antimicrobial Prophylaxis Influence the Diagnostic Potential of Periprosthetic Tissues in Hip or Knee Infections?. Clin Orthop Relat Res 474, 258–264 (2016). https://doi.org/10.1007/s11999-015-4486-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-015-4486-4