Abstract
Background
Adipose-derived stem cells have recently shown differentiation potential in multiple mesenchymal lineages in vitro and in vivo. These cells can be easily isolated in large amounts from autologous adipose tissue and used without culturing or differentiation induction, which may make them relatively easy to use for clinical purposes; however, their use has not been tested in a distraction osteogenesis model.
Question/purposes
The question of this animal study in a rodent model of distraction osteogenesis was whether uncultured adipose-derived regenerative cells (ADRCs), which can easily be isolated in large amounts from autologous adipose tissue and contain several types of stem and regenerative cells, promote bone formation in distraction osteogenesis. We evaluated this using several tools: (1) radiographic analysis of bone density; (2) histological analysis of the callus that formed; (3) biomechanical testing; (4) DiI labeling (a method of membrane staining for postimplant celltracing); and (5) real-time polymerase chain reaction.
Methods
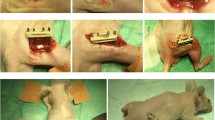
Sixty rats were randomly assigned to three groups. Physiological saline (control group), Type I collagen gel (collagen group), or a mixture of ADRC and Type I collagen gel (ADRC group) was injected into the distracted callus immediately after distraction termination. To a rat femur an external fixator was applied at a rate of 0.8 mm/day for 8 days.
Results
The bone density of the distracted callus in the ADRC group increased by 46% (p = 0.003, Cohen’s d = 10.2, 95% confidence interval [CI] ± 0.180) compared with the control group at 6 weeks after injection. The fracture strength in the ADRC group increased by 66% (p = 0.006, Cohen’s d = 1.32, 95% CI ± 0.180) compared with the control group at 6 weeks after injection. Real-time reverse transcription–polymerase chain reaction of the distracted callus from the ADRC group had higher levels of bone morphogenetic protein-2 (7.4 times higher), vascular endothelial growth factor A (6.8 times higher), and stromal cell-derived factor-1 (4.3 times higher). Cell labeling in the newly formed bone showed the ADRCs differentiated into osseous tissue at 3 weeks after injection.
Conclusions
The injection of ADRCs promoted bone formation in the distracted callus and this mechanism involves both osteogenic differentiation and secretion of humoral factors such as bone morphogenetic protein-2 or vascular endothelial growth factor A that promotes osteogenesis or angiogenesis.
Clinical Relevance
The availability of an easily accessible cell source may greatly facilitate the development of new cell-based therapies for regenerative medicine applications in the distraction osteogenesis.






Similar content being viewed by others
References
Ashton BA, Allen TD, Howlett CR, Eaglesom CC, Hattori A, Owen M. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res. 1980;151:294–307.
Eralp L, Ozkan K, Kocaoglu M, Aktas S, Zihni M, Türker M, Ozkan FU. Effects of hyperbaric oxygen therapy on distraction osteogenesis. Adv Ther. 2007;24:326–332.
Gimble JM, Katz AJ, Bunnel BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260.
Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–133.
Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part I: the influence of stability of fixation and soft tissue preservation. Clin Orthop Relat Res. 1989;238:249–281.
Ilizarov GA. The tension-stress effect on the genesis and growth of tissues. Part II: the influence of stability of the rate and frequency of distraction. Clin Orthop Relat Res. 1989;239:263–285.
Kawamoto T, Shimizu M. A method for preparing 2- to 50-micron-thick fresh-frozen sections of large samples and undecalcified hard tissues. Histochem Cell Biol. 2000;113:331–339.
Kitoh H, Kitakoji T, Tsuchiya H, Katoh M, Ishiguro N. Distraction osteogenesis of the lower extremity in patients with achondroplasia/hypochondroplasia treated with transplantation of culture-expanded bone marrow cells and platelet-rich plasma. J Pediatr Orthop. 2007;27:629–634.
Kondo K, Shintani S, Shibata R, Murakami H, Murakami R, Imaizumi M, Kitagawa Y, Murohara T. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:61–66.
Levi B, James AW, Nelson ER, Vistnes D, Wu B, Lee M, Gupta A, Longaker MT. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 2010;5:e11177.
Levi B, James AW, Wan DC, Glotzbach JP, Commons GW, Longaker MT. Regulation of human adipose-derived stromal cell osteogenic differentiation by insulin-like growth factor-1 and platelet-derived growth factor-alpha. Plast Reconstr Surg. 2010;126:41–52.
Nakanishi C, Nagaya N, Ohnishi S, Yamahara K, Takabatake S, Konno T, Hayashi K, Kawashiri MA, Tsubokawa T, Yamagishi M. Gene and protein expression analysis of mesenchymal stem cells derived from rat adipose tissue and bone marrow. Circ J. 2011;75:2260–2268.
Pepper JR, Herbert MA, Anderson JR, Bobechko WP. Effect of capacitive coupled electrical stimulation on regenerate bone. J Orthop Res. 1996;14:296–302.
Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298.
Sakurakichi K, Tsuchiya H, Uehara K, Yamashiro T, Tomita K, Azuma Y. Effects of timing of low-intensity pulsed ultrasound on distraction osteogenesis. J Orthop Res. 2004;22:395–403.
Shoji T, Ii M, Matsumoto T, Kawamoto A, Kwon SM, Kuroda T, Kuroda R, Kurosaka M, Asahara T. Local transplantation of human multipotent adipose-derived stem cells accelerates fracture healing via enhanced osteogenesis and angiogenesis. Lab Invest. 2010;90:637–649.
Sojo K, Sawaki Y, Hattori H, Mizutani H, Ueda M. Immunohistochemical study of vascular endothelial growth factor (VEGF) and bone morphogenetic protein-2, -4 (BMP-2, -4) on lengthened rat femurs. J Craniomaxillofac Surg. 2005;33:238–245.
Spiegl U, Pätzold R, Friederichs J, Hungerer S, Militz M, Bühren V. Clinical course, complication rate and outcome of segmental resection and distraction osteogenesis after chronic tibial osteitis. Injury. 2013;44:1049–1056.
Suganuma S, Tada K, Hayashi K, Takeuchi A, Sugimoto N, Ikeda K, Tsuchiya H. Uncultured adipose-derived regenerative cells promote peripheral nerve regeneration. J Orthop Sci. 2013;18:145–151.
Takamine Y, Tsuchiya H, Kitakoji T, Kurita K, Ono Y, Ohshima Y, Kitoh H, Ishiguro N, Iwata H. Distraction osteogenesis enhanced by osteoblastlike cells and collagen gel. Clin Orthop Relat Res. 2002;399:240–246.
Tsuchiya H, Abdel-Wanis ME, Sakurakichi K, Yamashiro T, Tomita K. Osteosarcoma around the knee: intraepiphyseal excision and biological reconstruction with distraction osteogenesis. J Bone Joint Surg Br. 2002;84:1162–1166.
Tsuchiya H, Tomita K. Distraction osteogenesis for treatment of bone loss in the lower extremity. J Orthop Sci. 2003;8:116–124.
Tsuchiya H, Tomita K, Minematsu K, Mori Y, Asada N, Kitano S. Limb salvage using distraction osteogenesis: a classification of the technique. J Bone Joint Surg Br. 1997;79:403–411.
Vilalta M, Dégano IR, Bago J, Gould D, Santos M, García-Arranz M, Ayats R, Fuster C, Chernajovsky Y, García-Olmo D, Rubio N, Blanco J. Biodistribution, long-term survival, and safety of human adipose tissue-derived mesenchymal stem cells transplanted in nude mice by high sensitivity non-invasive bioluminescence imaging. Stem Cells Dev. 2008;17:993–1003.
Wan DC, Shi YY, Nacamuli RP, Quarto N, Lyons KM, Longaker MT. Osteogenic differentiation of mouse adipose-derived adult stromal cells requires retinoic acid and bone morphogenetic protein receptor type IB signaling. Proc Natl Acad Sci U S A. 2006;103:12335–12340.
Wolbank S, Peterbauer A, Wassermann E, Hennerbichler S, Voglauer R, van Griensven M, Duba HC, Gabriel C, Redl H. Labelling of human adipose-derived stem cells for non-invasive in vivo cell tracking. Cell Tissue Bank. 2007;8:163–177.
Xu Y, Hammerick KE, James AW, Carre AL, Leucht P, Giaccia AJ, Longaker MT. Inhibition of histone deacetylase activity in reduced oxygen environment enhances the osteogenesis of mouse adipose-derived stromal cells. Tissue Eng Part A. 2009;15:3697–3707.
Yasui N, Sato M, Ochi T, Kimura T, Kawahata H, Kitamura Y, Nomura S. Three modes of ossification during distraction osteogenesis in the rat. J Bone Joint Surg Br. 1997;79:824–830.
Yoshitake F, Itoh S, Narita H, Ishihara K, Ebisu S. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-kappaB signaling pathways. J Biol Chem. 2008;283:11535–11540.
Zhu M, Zhou Z, Chen Y, Schreiber R, Ransom JT, Fraser JK, Hedrick MH, Pinkernell K, Kuo HC. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg. 2010;64:222–228.
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228.
Acknowledgments
We thank Ms Yoko Kasai for her skillful technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
About this article
Cite this article
Nomura, I., Watanabe, K., Matsubara, H. et al. Uncultured Autogenous Adipose-derived Regenerative Cells Promote Bone Formation During Distraction Osteogenesis in Rats. Clin Orthop Relat Res 472, 3798–3806 (2014). https://doi.org/10.1007/s11999-014-3608-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-014-3608-8