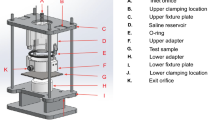
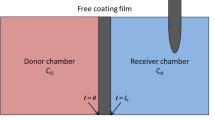
Abstract
For corrosion protective coatings that are designed to give lifetimes of protection that may extend to 50 years, valid accelerated test methods are necessary to develop improved systems and validate performance. Fluid flow over metals has long been believed to influence the corrosion process. Studies have been focused on the effects of flow rate on the corrosion of bare metals. The influence of fluid flow on the degradation of metal-protective coatings has received less attention. This paper describes a preliminary study on the influence of laminar flow on organic coatings. A Hele-Shaw cell and its associated fluid control apparatuses are incorporated into the electrochemical cell setup. The barrier properties of the coating as a function of immersion time and flow rate have been monitored by electrochemical impedance spectroscopy. We observe that the barrier properties of the coating measured electrochemically decrease exponentially with the increasing flow rate. We propose that the flowing electrolyte solution could be used in acceleration tests for the lifetime prediction of organic coatings as the acceleration of failure we have observed does not appear to change the mechanism of failure. Further analysis is proposed to validate immersion flow rate as a universal accelerating parameter for coating failure.










Similar content being viewed by others
Notes
Mills and Bierwagen did some initial work on examining flow effects as an acceleration factor for corrosion in coated systems (NDSU 1994, Unpublished work).
The coatings are from KCC Corporation (http://www.kccworld.co.kr) and graciously supplied by Dohn Lee.
References
Heitz, E, “Mechanistically based prevention strategies of flow-induced corrosion.” Electrochimica Acta 41 503–509 (1996). doi:10.1016/0013-4686(95)00336-3..
Mercer, D, Lumbard, EA, “Corrosion of mild steel in water.” British Corrosion Journal 30 43–55 (1995).
Wharton, JA, Wood, RJK, “Influence of flow conditions on the corrosion of AISI 304L stainless steel.” Wear 256 525–536 (2004). doi:10.1016/S0043-1648(03)00562-3.
Free, ML, “Mathematical Modeling and Experimental Validation of Steel and Copper Corrosion Based Upon Combined Thermodynamic, Electrochemical, and Mass Transport Fundamentals.” Tri-Service Corrosion Conference, Orlando, FL, November 2005
Melchers, RE, Jeffrey, R, “Influence of water velocity on marine immersion corrosion of mild steel.” Corrosion 60 84–94 (2004).
Heeg, B, Moros, T, Klenerman, D, “Persistency of corrosion inhibitor films on C-steel under multiphase flow conditions Part I: the jet-cylinder arrangement.” Corrosion Science 40 1303–1311(1998). doi:10.1016/S0010-938X(98)00012-2.
Zeller III RL, “Electrochemical corrosion testing of high phosphorus electroless Nickel in 5% NaCl.” Corrosion 47 692–702 (1991).
Hong, T, Chen, Y, Sun, YH, Jepson, WP, “Monitoring corrosion in multiphase pipelines.” Materials and Corrosion 52 590–597 (2001). doi:10.1002/1521-4176(200108)52:8<590::AID-MACO590>3.0.CO;2-4
Chen, Y, Hong, T, Gopal, M, Jepson, WP, “EIS studies of a corrosion inhibitor behavior under multiphase flow conditions.” Corrosion Science 42 979–990 (2000). doi:10.1016/S0010-938X(99)00127-4.
Ruzic, V, Veidt, M, Nesic, S, “Protective iron carbonate films – Part 1: mechanical removal in single-phase aqueous flow.” Corrosion 62 419–432 (2006).
Jeffcoate, CS, Bierwagen, GP, “Initial Studies of Electrochemical Comparison of Coating Performance in Flowing Versus Stationary Electrolyte.” In: Bierwagen, GP (ed.) Organic Coatings for Corrosion Control, pp. 151–160. ACS Symposium Series 689, American Chemical Society, Washington, DC (1998)
Wei, YH, Zhang, LX, Ke, W, “Comparison of the degradation behavior of fusion-bonded epoxy powder coating systems under flowing and static immersion.” Corrosion Science 48 1449–1461 (2006). doi:10.1016/j.corsci.2005.05.016.
De Rosa, L, Monetta, T, Mitton DB, Bellucci, F, “Monitoring degradation of single and multilayer organic coatings. I. Absorption and transport of water: theoretical analysis and methods.” J. Electrochem. Soc. 145 3830–3838 (1998). doi:10.1149/1.1838881.
Hu, JM, Zhang, JQ, Cao, CN, “Determination of water uptake and diffusion of Cl− ion in epoxy primer on aluminum alloys in NaCl solution by electrochemical impedance spectroscopy.” Prog. in Organic Coatings 46 273–279 (2003). doi:10.1016/S0300-9440(03)00010-9.
Zhang, JT, Hu, JM, Zhang, JQ, Cao, CN, “Studies of impedance models and water transport behaviors of polypropylene coated metals in NaCl solution.” Prog. in Organic Coatings 49 293–301 (2004). doi:10.1016/S0300-9440(03)00115-2.
Hinderliter, BR, Croll, SG, Tallman, DE, Su, Q, Bierwagen, GP, “Interpretation of EIS data from accelerated exposure of coated metals based on modeling of coating physical properties.” Electrochimica Acta 51 4505–4515 (2006). doi:10.1016/j.electacta.2005.12.047.
Stafford, OA, Hinderliter, BR, and Croll, SG, “Electrochemical impedance spectroscopy response of water uptake in organic coatings by finite element methods.” Electrochimica Acta 52 1339–1348 (2006). doi:10.1016/j.electacta.2006.07.047.
Allahar, KN, Hinderliter, BR, Simoes, AM, Tallman, DE, Bierwagen, GP, Croll, SG, “Simulation of wet−dry cycling of organic coatings using ionic liquids.” J. Electrochem. Soc. 154 F177-F185 (2007). doi:10.1149/1.2764235.
Allahar, KN, Hinderliter, BR, Bierwagen, GP, Tallman DE, and Croll, SG, “Cyclic wet drying of an epoxy coating using an ionic liquid.” Progress in Organic Coatings, 62 87–95 (2008). doi:10.1016/j.porgcoat.2007.09.018.
Allahar, KN, Hinderliter, BR, Tallman, DE, and Bierwagen, GP, “Water transport in multilayer organic coatings.” J. Electrochem. Soc. 155 F201- F208 (2008). doi:10.1149/1.2946429.
Bierwagen, GP, He, L, Li, J, Ellingson, L, Tallman, DE, “Studies of a new accelerated evaluation method for coating corrosion resistance—thermal cycling testing.” Prog. Organic Coatings 39 67–78 (2000). doi:10.1016/S0300-9440(00)00106-5.
Su, Q, Allahar, KN, Bierwagen, GP, “Application of embedded sensors in the thermal cycling of organic coatings.” Corrosion Science 50 2381–2389 (2008). doi:10.1016/j.corsci.2008.06.010.
Hinderliter, BR, Allahar, KN, Bierwagen, GP, Tallman, DE, and Croll, SG, “Thermal Cycling of Epoxy Coatings Using Room Temperature Ionic Liquids,” J. Electrochem.Soc. 155 C93–100 (2008). doi:10.1149/1.2822991.
Acknowledgments
The authors would like to thank the US Air Force Office of Scientific Research for support of this research. This work is supported under the Contract Number: FA9550-04-1-0368.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Bierwagen, G.P. A new acceleration factor for the testing of corrosion protective coatings: flow-induced coating degradation. J Coat Technol Res 6, 429–436 (2009). https://doi.org/10.1007/s11998-008-9161-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-008-9161-1