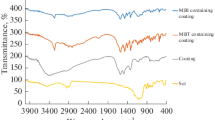
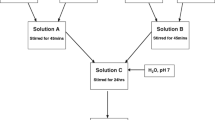
Abstract
New coatings have been developed that display resistance to the nucleation and adhesion of ice [Simendinger, WH III, Miller, SD, Anti-icing composition. US Patent #6,702,953 2004]. These coatings contain a titanium alkoxide-based sol–gel system designed to facilitate the slow release of tripropylene glycol (TPG) and glycerol, which depress the freezing point of water. The performance and the lifetime of this coating critically depend on the rate at which TPG and glycerol are released to the coating surface. The kinetics and mechanism of this process are studied in this article. Mass loss measurements are reported for temperatures ranging from 22 to 90°C for both the isolated sol–gel and the coating. Two regions of mass loss are observed: loss of isopropyl alcohol, which begins immediately after the reactants are mixed, and loss of TPG and glycerol, which begins at a later time, extending up to several months at low temperatures. Diffusivities and activation energies for IPA and TPG/glycerol in the sol–gel are obtained from 22 to 90°C and compared to similar data obtained for an anti-icing coating containing the dispersed sol–gel. The effect of changing the sol–gel reaction conditions on mass loss kinetics is also reported.











Similar content being viewed by others
References
Simendinger, WH III, Miller, SD, “Anti-icing composition.” US Patent #6,702,953, 2004
Brinker, JC, Scherer, GW, Sol Gel Science: The Physics and Chemistry of Sol-Gel Processing. Boston Academic Press (1990)
Livage, J, Henry, M, Sanchez, J, “Sol-gel Chemistry of Transition Metal Oxides.” Prog. Solid State Chem., 18 259–341 (1988)
Livage, J, In: Aegerter, MA, Jafelicci Jr, M, de Souza, DF, Zanotto, ED (eds.) Sol-Gel Science and Technology. World Scientific Publishing, 1989
Mackenzie, JD, In: Sakke, S, Klein Lisa, C (ed.) Sol-Gel Science and Technology. American Ceramic Society (1995)
Narula, CK, Ceramic Precursor Technology and Its Applications. Marcel Dekker, Inc. (1995)
Wright, JD, Sommerdijk, NAJM (2001) Sol-Gel Materials, Chemistry and Applications. Gordon and Breach Science Publishers
Zarzycki J (1997) Past and Present of Sol-Gel Science and Technology. J. Sol-Gel Sci. Technol. 8:17–22
Ayres, J, Simendinger, WH, III, Balik, CM, “Characterization of Titanium Alkoxide Sol-Gel Systems Designed for Anti-Icing Coatings: I. Chemistry.” J. Coat. Technol. Res. in press (2007)
Livage, J, Henry, M, “Predictive Model for Inorganic Polymerization.” In: MacKenzie, JD, Ulrich, DR (eds.) Reaction Ultrastructure Processing of Advanced Ceramics, pp. 183–195. Wiley & Sons, New York (1988)
Balik, CM, “On the Extraction of Diffusion Coefficients from Gravimetric Data for Sorption of Small Molecules by Polymer Thin Films.” Macromolecules, 29 3025–3029 (1996)
Chang I, Sillescu HJ (1997) Heterogeneity at the Glass Transition: Translational and Rotational Self-Diffusion. J. Phys. Chem. B 101(43):8794–8801
Mensetieri G, Lavorgna M, Mustro P, Ragosta G (2006) Water transport in densely crosslinked networks: A comparison between epoxy systems having different interactive characters. Polymer 47:8326–8336
Pauly, S, In: Brandrup, J, Immergut, EH (eds.) Polymer Handbook, 3rd ed., pp. VI 435–VI 461. Wiley & Sons, New York (1989)
Pennarun PY, Ngono Y, Dole P, Feigenbaum AJ (2004) Functional barriers in PET recycled bottles. Part II. Diffusion of pollutants during processing. J. Appl. Polymer Sci. 92:2859–2870
Acknowledgments
The authors gratefully acknowledge Microphase Coatings, Inc. for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ayres, J., Simendinger, W.H. & Balik, C.M. Characterization of titanium alkoxide sol–gel systems designed for anti-icing coatings: II. Mass loss kinetics. J Coat Technol Res 4, 473–481 (2007). https://doi.org/10.1007/s11998-007-9055-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-007-9055-7