Abstract
Corn pericarp is a low-value byproduct of the processing industry. However, due to a disproportionately higher concentration of phytochemicals, colored corn pericarp could be a source of nutraceuticals and food additives. For the first time, purple corn pericarp (PCP) was converted to a polyphenol-rich extract containing anthocyanins, phenolic acids, and proanthocyanins using a two-pot microwave extraction technique. Besides, the microwave extraction (MAE) conditions were optimized, and response surface methodology was used to understand the association between independent variables and their responses and used further to decipher the underlying mechanisms through visualization. Plackett–Burman design (PBD) was used to screen significant extraction parameters, and further optimization was done using Box-Behnken design (BBD). Under the optimum conditions (ethanol (42.4% v/v), temperature (75 °C), and time (29 min)), total anthocyanin content (TAC), total phenolic content (TPC), and condensed tannins (CT) to the tune of 38.73 g/kg, 138.62 g/kg and 279.48 g/kg pericarp, respectively were obtained with a desirability function value of 0.838. Monomeric anthocyanins degraded and polymerized to 3-deoxyanthocyanin, whereas phenolic acids such as chlorogenic acid, caffeic acid, ferulic acid, and hesperidin increased as the microwave temperature and time increased. The MAE’s extraction yield was 38.11% higher than the conventional extraction (CE). The CE process took ~ 8.6 h, whereas MAE took ~ 0.5 h to extract the phenolics. The MAE samples had a higher TAC, TPC, CT, phenolic acids (chlorogenic acid, caffeic acid, ferulic acid, and hesperidin), total flavonoid content (TFC), and antioxidant activities than CE samples. Therefore, the valorization of PCP could contribute to the circular economy model.
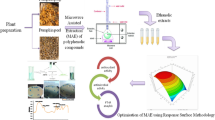
Graphical Abstract








Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CE:
-
Conventional extraction
- RSM:
-
Response surface methodology
- BBD:
-
Box-Behnken design
- DPPH:
-
1,2-Diphenylpicrylhydrazyl
- PCPP:
-
Purple corn pericarp powder
- C3G:
-
Cyanidin-3-O-glucoside
- TFC:
-
Total flavonoid content
- CE:
-
Catechin equivalent
- L* :
-
Lightness
- b* :
-
Yellowness to blueness
- ΔE :
-
Total color difference
- WSI:
-
Water solubility index
- PCPE:
-
Purple corn pericarp extract
- PCPE:
-
Scanning electron microscopy
- ZP:
-
Zeta potential
- EE:
-
Epicatechin equivalent
- LoF:
-
Lack of fit
- MAE:
-
Microwave-assisted extraction
- PBD:
-
Plackett- Burman design
- TAC:
-
Total anthocyanin content
- ABTS:
-
2,2-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)
- HPLC:
-
High-performance liquid chromatography
- TPC:
-
Total phenolic content
- CT:
-
Condensed tannins
- EY:
-
Extraction yield
- a* :
-
Greenness to redness
- C :
-
Chroma
- H o :
-
Hue angle
- WAI:
-
Water absorption index
- FTIR:
-
Fourier transform infrared
- PSD:
-
Particle size distribution
- PDI:
-
Poly dispersibility index,
- CUPRAC:
-
Cupric ion reducing antioxidant capacity
- TA:
-
Titrable acidity
References
Adams, J. B. (1973). Thermal degradation of anthocyanins with particular reference to the 3-glycosides of cyanidin. I. In acidified aqueous solution at 100 °C. Journal of the Science of Food and Agriculture, 24(7), 747–762. https://doi.org/10.1002/JSFA.2740240702
Adainoo, B., Thomas, A. L., & Krishnaswamy, K. (2023). Correlations between color, textural properties and ripening of the North American pawpaw (Asimina triloba) fruit. Sustainable Food Technology. https://doi.org/10.1039/D2FB00008C
Ahmadiani, N., Robbins, R. J., Collins, T. M., & Giusti, M. M. (2014). Anthocyanins contents, profiles, and color characteristics of red cabbage extracts from different cultivars and maturity stages. Journal of Agricultural and Food Chemistry, 62(30), 7524–7531. https://doi.org/10.1021/jf501991q
Akpabli-Tsigbe, N. D. K., Ma, Y., Ekumah, J. N., Osabutey, J., Hu, J., Xu, M., & Johnson, N. A. N. (2021a). Novel solid-state fermentation extraction of 5-O-caffeoylquinic acid from heilong48 soybean using Lactobacillus helviticus: Parametric screening and optimization. Lwt, 149(March), 111809. https://doi.org/10.1016/j.lwt.2021.111809
Akpabli-Tsigbe, N. D. K., Ma, Y., Ekumah, J. N., Osabutey, J., Hu, J., Xu, M., Johnson, N. A. N., & Mintah, B. K. (2022). Ultrasonic-assisted extraction of bioactive chlorogenic acid from heilong48 soybean variety: Parametric optimization and evaluation of physicochemical and bioactive properties. Food Science and Nutrition, 10(4), 985–1002. https://doi.org/10.1002/fsn3.2670
Akpabli-Tsigbe, N. D. K., Ma, Y., Ekumah, J. N., Osabutey, J., Hu, J., Xu, M., Johnson, N. A. N., & Quaisie, J. (2021b). Two-step optimization of solid-state fermentation conditions of heilong48 soybean variety for maximum chlorogenic acid extraction yield with improved antioxidant activity. Industrial Crops and Products, 168(January), 113565. https://doi.org/10.1016/j.indcrop.2021.113565
Alolga, R. N., Osae, R., Essilfie, G., Saalia, F. K., Akaba, S., & Chikari, F. (2021). Sonication, osmosonication and vacuum-assisted osmosonication pretreatment of Ghanaian garlic slices: Effect on physicochemical properties and quality characteristics. Food Chemistry, 343(October 2020), 128535. https://doi.org/10.1016/j.foodchem.2020.128535
Ameer, K., Shahbaz, H. M., & Kwon, J. H. (2017). Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Comprehensive Reviews in Food Science and Food Safety, 16(2), 295–315. https://doi.org/10.1111/1541-4337.12253
Bagade, S. B., & Patil, M. (2021). Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Critical Reviews in Analytical Chemistry, 51(2), 138–149. https://doi.org/10.1080/10408347.2019.1686966
Bezerra, M. A., Ferreira, S. L. C., Novaes, C. G., dos Santos, A. M. P., Valasques, G. S., da Mata Cerqueira, U. M. F., & dos Santos Alves, J. P. (2019). Simultaneous optimization of multiple responses and its application in Analytical Chemistry – A review. Talanta, 194(October 2018), 941–959. https://doi.org/10.1016/j.talanta.2018.10.088
Boakye, P. G., Ismail, B. P., & Annor, G. A. (2022). Optimizing the extrusion conditions for the production of expanded intermediate wheatgrass ( Thinopyrum intermedium ) products. Journal of Food Science, December 2021, 1–17. https://doi.org/10.1111/1750-3841.16238
Boateng, I. D., & Yang, X. (2021a). Do non-thermal pretreatments followed by intermediate-wave infrared drying affect toxicity, allergenicity, bioactives, functional groups, and flavor components of Ginkgo biloba seed ? A case study. Industrial Crops & Products, 165, 113421. https://doi.org/10.1016/j.indcrop.2021.113421
Boateng, I. D., & Yang, X. (2021b). Thermal and non-thermal processing affect Maillard reaction products, flavor, and phytochemical profiles of Ginkgo biloba seed. Food Bioscience, 41, 101044. https://doi.org/10.1016/j.fbio.2021.101044
Boateng, I. D., & Yang, X. M. (2021c). Process optimization of intermediate-wave infrared drying: Screening by Plackett–Burman; comparison of Box-Behnken and central composite design and evaluation: A case study. Industrial Crops and Products, 162(August 2020), 113287. https://doi.org/10.1016/j.indcrop.2021.113287
Boateng, I. D., Yang, X. M., & Li, Y. Y. (2020). Optimization of infrared-drying parameters for Ginkgo biloba L. seed and evaluation of product quality and bioactivity. Industrial Crops and Products, 160(October 2020), 113108. https://doi.org/10.1016/j.indcrop.2020.113108
Boateng, I. D., Yang, X. M., Tahany, A. A. A., Li, Y. Y., & Yolandani. (2021). Drying methods affect organoleptic and physicochemical properties of rehydrated ginkgo seed slices. Industrial Crops and Products, 160(July 2020), 113166. https://doi.org/10.1016/j.indcrop.2020.113166
Boateng, I. D., Zhang, W., Li, Y., Saalia, F. K., & Yang, X. (2022). Non-thermal pretreatment affects Ginkgo biloba L. seed ’ s product qualities, sensory, and physicochemical properties. Journal of Food Science, December, 1–18. https://doi.org/10.1111/1750-3841.15999
Bosso, A., Guaita, M., & Petrozziello, M. (2016). Influence of solvents on the composition of condensed tannins in grape pomace seed extracts. Food Chemistry, 207, 162–169. https://doi.org/10.1016/j.foodchem.2016.03.084
Carniel, N., Dallago, R. M., Dariva, C., Bender, J. P., Nunes, A. L., Zanella, O., Bilibio, D., & Luiz Priamo, W. (2017). Microwave-Assisted Extraction of Phenolic Acids and Flavonoids from Physalis angulata. Journal of Food Process Engineering, 40(3), 1–11. https://doi.org/10.1111/jfpe.12433
Cevallos-Casals, B. A., & Cisneros-Zevallos, L. (2003). Stoichiometric and kinetic studies of phenolic antioxidants from Andean purple corn and red-fleshed sweetpotato. Journal of Agricultural and Food Chemistry, 51(11), 3313–3319. https://doi.org/10.1021/jf034109c
Chan, C. H., Yusoff, R., Ngoh, G. C., & Kung, F. W. L. (2011). Microwave-assisted extractions of active ingredients from plants. Journal of Chromatography A, 1218(37), 6213–6225. https://doi.org/10.1016/j.chroma.2011.07.040
Chaves, J. O., de Souza, M. C., da Silva, L. C., Lachos-Perez, D., Torres-Mayanga, P. C., Machado, A. P. da F., Forster-Carneiro, T., Vázquez-Espinosa, M., González-de-Peredo, A. V., Barbero, G. F., & Rostagno, M. A. (2020). Extraction of Flavonoids From Natural Sources Using Modern Techniques. Frontiers in Chemistry, 8(September). https://doi.org/10.3389/fchem.2020.507887
Chen, X. M., Ma, Z., & Kitts, D. D. (2018). Effects of processing method and age of leaves on phytochemical profiles and bioactivity of coffee leaves. Food Chemistry, 249, 143–153. https://doi.org/10.1016/j.foodchem.2017.12.073
Cristianini, M., & Guillén Sánchez, J. S. (2020). Extraction of bioactive compounds from purple corn using emerging technologies: A review. Journal of Food Science, 85(4), 862–869. https://doi.org/10.1111/1750-3841.15074
Dahmoune, F., Nayak, B., Moussi, K., Remini, H., & Madani, K. (2015). Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chemistry, 166, 585–595. https://doi.org/10.1016/j.foodchem.2014.06.066
Das, A. K., & Singh, V. (2016). Antioxidative free and bound phenolic constituents in botanical fractions of Indian specialty maize (Zea mays L.) genotypes. Food Chemistry, 201, 298–306. https://doi.org/10.1016/j.foodchem.2016.01.099
de Pascual-Teresa, S., & Sanchez-Ballesta, M. T. (2008). Anthocyanins: From plant to health. Phytochemistry Reviews, 7, 281–299. https://doi.org/10.1007/s11101-007-9074-0
Dhanani, T., Shah, S., Gajbhiye, N. A., & Kumar, S. (2017). Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arabian Journal of Chemistry, 10, S1193–S1199. https://doi.org/10.1016/j.arabjc.2013.02.015
Doulabi, M., Golmakani, M. T., & Ansari, S. (2020). Evaluation and optimization of microwave-assisted extraction of bioactive compounds from eggplant peel by-product. Journal of Food Processing and Preservation, 44(11), 1–13. https://doi.org/10.1111/jfpp.14853
Durazzo, A., Kiefer, J., Lucarini, M., Camilli, E., Marconi, S., Gabrielli, P., Aguzzi, A., Gambelli, L., Lisciani, S., & Marletta, L. (2018). Qualitative analysis of traditional Italian dishes: FTIR approach. Sustainability, 10(11). https://doi.org/10.3390/su10114112
Dzah, C. S., Duan, Y., Zhang, H., Authur, D. A., & Ma, H. (2020a). Ultrasound-, subcritical water- and ultrasound assisted subcritical water-derived Tartary buckwheat polyphenols show superior antioxidant activity and cytotoxicity in human liver carcinoma cells. Food Research International, 137, 109598. https://doi.org/10.1016/j.foodres.2020.109598
Dzah, C. S., Duan, Y., Zhang, H., Serwah Boateng, N. A., & Ma, H. (2020b). Latest developments in polyphenol recovery and purification from plant by-products: A review. Trends in Food Science and Technology, 99(October 2019), 375–388. https://doi.org/10.1016/j.tifs.2020.03.003
Fakayode, O. A., Aboagarib, E. A. A., Yan, D., Li, M., Wahia, H., Mustapha, A. T., Zhou, C., & Ma, H. (2020). Novel two-pot approach ultrasonication and deep eutectic solvent pretreatments for watermelon rind delignification: Parametric screening and optimization via response surface methodology. Energy, 203. https://doi.org/10.1016/j.energy.2020.117872
Ferreira, S. L. C., Silva Junior, M. M., Felix, C. S. A., da Silva, D. L. F., Santos, A. S., Santos Neto, J. H., de Souza, C. T., Cruz Junior, R. A., & Souza, A. S. (2019). Multivariate optimization techniques in food analysis – A review. Food Chemistry, 273(November 2017), 3–8. https://doi.org/10.1016/j.foodchem.2017.11.114
Gao, N., Sun, X., Li, D., Gong, E., Tian, J., Si, X., Jiao, X., Xing, J., Wang, Y., Meng, X., & Li, B. (2020). Optimization of anthocyanidins conversion using chokeberry pomace rich in polymeric proanthocyanidins and cellular antioxidant activity analysis. Lwt, 133(July). https://doi.org/10.1016/j.lwt.2020.109889
García-Márquez, E., Higuera-Ciapara, I., & Espinosa-Andrews, H. (2017). Design of fish oil-in-water nanoemulsion by microfluidization. Innovative Food Science and Emerging Technologies, 40, 87–91. https://doi.org/10.1016/j.ifset.2016.11.007
Gil-Martín, E., Forbes-Hernández, T., Romero, A., Cianciosi, D., Giampieri, F., & Battino, M. (2022). Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chemistry, 378. https://doi.org/10.1016/j.foodchem.2021.131918
Granato, D., & Ares, G. (2014). Mathematical and Statistical Methods in Food Science and Technology (First). John Wiley & Sons, Ltd. https://doi.org/10.1002/9781118434635
Haya, S., Bentahar, F., & Trari, M. (2019). Optimization of polyphenols extraction from orange peel. Journal of Food Measurement and Characterization, 13(1), 614–621. https://doi.org/10.1007/s11694-018-9974-2
Hayat, K., Zhang, X., Farooq, U., Abbas, S., Xia, S., Jia, C., Zhong, F., & Zhang, J. (2010). Effect of microwave treatment on phenolic content and antioxidant activity of citrus mandarin pomace. Food Chemistry, 123(2), 423–429. https://doi.org/10.1016/j.foodchem.2010.04.060
Herrman, D. A., Brantsen, J. F., Ravisankar, S., Lee, K. M., & Awika, J. M. (2020a). Stability of 3-deoxyanthocyanin pigment structure relative to anthocyanins from grains under microwave assisted extraction. Food Chemistry, 333(July), 127494. https://doi.org/10.1016/j.foodchem.2020.127494
Herrman, D. A., Brantsen, J. F., Ravisankar, S., Lee, K. M., & Awika, J. M. (2020b). Stability of 3-deoxyanthocyanin pigment structure relative to anthocyanins from grains under microwave assisted extraction. Food Chemistry, 333(July), 127494. https://doi.org/10.1016/j.foodchem.2020.127494
Hutabarat, R. P., Xiao, Y. D., Wu, H., Wang, J., Li, D. J., & Huang, W. Y. (2019). Identification of anthocyanins and optimization of their extraction from rabbiteye blueberry fruits in Nanjing. Journal of Food Quality, 2019. https://doi.org/10.1155/2019/6806790
Jafari, S. M., Mahdavee Khazaei, K., & Assadpour, E. (2019). Production of a natural color through microwave-assisted extraction of saffron tepal’s anthocyanins. Food Science and Nutrition, 7(4), 1438–1445. https://doi.org/10.1002/fsn3.978
Kandil, A., Li, J., Vasanthan, T., & Bressler, D. C. (2012). Phenolic acids in some cereal grains and their inhibitory effect on starch liquefaction and saccharification. Journal of Agricultural and Food Chemistry, 60(34), 8444–8449. https://doi.org/10.1021/jf3000482
Karabegović, I. T., Stojičević, S. S., Veličković, D. T., Nikolić, N. Č, & Lazić, M. L. (2013). Optimization of microwave-assisted extraction and characterization of phenolic compounds in cherry laurel (Prunus laurocerasus) leaves. Separation and Purification Technology, 120, 429–436. https://doi.org/10.1016/j.seppur.2013.10.021
Kashyap, P., Riar, C. S., & Navdeep, J. (2018). Intensification of Polyphenols Extraction from Sohiong (Prunus nepalensis) using Microwave-Assisted Extraction. Asian Journal of Chemistry, 30(8), 1717–1722.
Khoo, H. E., Azlan, A., Tang, S. T., & Lim, S. M. (2017). Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food and Nutrition Research, 61(1), 0–21. https://doi.org/10.1080/16546628.2017.1361779
Kyere-Yeboah, K., & Qiao, X. C. (2023). Process optimization of dielectric barrier discharge reactor for chloroform degradation using central composite design. Chemical Engineering Communications, 1–16. https://doi.org/10.1080/00986445.2023.2172571
Laishram, B., & Das, A. B. (2017). Effect of thermal pretreatments on physical, phytochemical, and antioxidant properties of black rice pasta. Journal of Food Process Engineering, 40(5), 1–8. https://doi.org/10.1111/jfpe.12553
Lao, F., & Giusti, M. M. (2016). Quantification of Purple Corn (Zea mays L.) Anthocyanins Using Spectrophotometric and HPLC Approaches: Method Comparison and Correlation. Food Analytical Methods, 9(5), 1367–1380. https://doi.org/10.1007/s12161-015-0318-0
Lao, F., & Giusti, M. M. (2018). Extraction of purple corn (Zea mays L.) cob pigments and phenolic compounds using food-friendly solvents. Journal of Cereal Science, 80, 87–93. https://doi.org/10.1016/j.jcs.2018.01.001
Lao, F., Sigurdson, G. T., & Giusti, M. M. (2017). Health Benefits of Purple Corn (Zea mays L.) Phenolic Compounds. Comprehensive Reviews in Food Science and Food Safety, 16(2), 234–246. https://doi.org/10.1111/1541-4337.12249
Lee, J., Durst, R. W., & Wrolstad, R. E. (2005). Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. Journal of AOAC International, 88(5), 1269–1278. https://doi.org/10.1093/jaoac/88.5.1269
Lefebvre, T., Destandau, E., & Lesellier, E. (2021). Selective extraction of bioactive compounds from plants using recent extraction techniques: A review. Journal of Chromatography A, 1635, 461770. https://doi.org/10.1016/j.chroma.2020.461770
Li, H., Deng, Z., Wu, T., Liu, R., Loewen, S., & Tsao, R. (2012). Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chemistry, 130(4), 928–936. https://doi.org/10.1016/j.foodchem.2011.08.019
Li, X., Wei, Y., Xu, J., Xu, N., & He, Y. (2018). Quantitative visualization of lignocellulose components in transverse sections of moso bamboo based on FTIR macro- and micro-spectroscopy coupled with chemometrics. Biotechnology for Biofuels, 11(1), 1–16. https://doi.org/10.1186/s13068-018-1251-4
Liu, Z., Li, H., Qi, Y., Zhu, Z., Huang, D., Zhang, K., Pan, J., Wen, L., & Zou, Z. (2021). Cinnamomum camphora leaves as a source of proanthocyanidins separated using microwave-assisted extraction method and evaluation of their antioxidant activity in vitro. Arabian Journal of Chemistry, 14(9), 103328. https://doi.org/10.1016/j.arabjc.2021.103328
Montgomery, D. C. (2017). Design and Analysis of Experiments. In John Wiley & Sons, Inc (9th ed., Vol. 9). John Wiley & Sons, Inc. https://www.wiley.com/en-ie/Design+and+Analysis+of+Experiments,+9th+Edition,+EMEA+Edition-p-9781119638421
Nani, M., & Krishnaswamy, K. (2022). Circular economy for food industry waste: Development and characterization of spray-dried acid whey encapsulated in millet matrix. ACS Food Science & Technology. https://doi.org/10.1021/acsfoodscitech.2c00326
Ngamwonglumlert, L., & Devahastin, S. (2017). Microstructure and its relationship with quality and storage stability of dried foods. Elsevier Ltd. https://doi.org/10.1016/b978-0-08-100764-8.00008-3
Oh, Y. S., Lee, J. H., Yoon, S. H., Oh, C. H., Choi, D. S., Choe, E., & Jung, M. Y. (2008). Characterization and quantification of anthocyanins in grape juices obtained from the grapes cultivated in Korea by HPLC/DAD, HPLC/MS, and HPLC/MS/MS. Journal of Food Science, 73(5), 378–389. https://doi.org/10.1111/j.1750-3841.2008.00756.x
Ongkowijoyo, P., Luna-Vital, D. A., & Gonzalez de Mejia, E. (2018). Extraction techniques and analysis of anthocyanins from food sources by mass spectrometry: An update. Food Chemistry, 250(January), 113–126. https://doi.org/10.1016/j.foodchem.2018.01.055
Özbek, H. N., Yanık, D. K., Fadıloğlu, S., & Fahrettin, G. (2020). Optimization of microwave-assisted extraction of bioactive compounds from pistachio (Pistacia vera L.) hull. Separation Science and Technology (Philadelphia), 55(2), 289–299. https://doi.org/10.1080/01496395.2019.1577444
Pap, N., Beszédes, S., Pongrácz, E., Myllykoski, L., Gábor, M., Gyimes, E., Hodúr, C., & Keiski, R. L. (2013). Microwave-Assisted Extraction of Anthocyanins from Black Currant Marc. Food and Bioprocess Technology, 6(10), 2666–2674. https://doi.org/10.1007/s11947-012-0964-9
Patras, A., Brunton, N. P., O’Donnell, C., & Tiwari, B. K. (2010). Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends in Food Science and Technology, 21(1), 3–11. https://doi.org/10.1016/j.tifs.2009.07.004
Peniche-Pavía, H. A., & Tiessen, A. (2020). Anthocyanin Profiling of Maize Grains Using DIESI-MSQD Reveals That Cyanidin-Based Derivatives Predominate in Purple Corn, whereas Pelargonidin-Based Molecules Occur in Red-Pink Varieties from Mexico. Journal of Agricultural and Food Chemistry, 68(21), 5980–5994. https://doi.org/10.1021/acs.jafc.9b06336
Pérez-Gregorio, R. M., García-Falcón, M. S., Simal-Gándara, J., Rodrigues, A. S., & Almeida, D. P. F. (2010). Identification and quantification of flavonoids in traditional cultivars of red and white onions at harvest. Journal of Food Composition and Analysis, 23(6), 592–598. https://doi.org/10.1016/j.jfca.2009.08.013
Ramos-Escudero, F., Muñoz, A. M., Alvarado-Ortíz, C., Alvarado, Á., & Yáñez, J. A. (2012). Purple corn (Zea mays L.) phenolic compounds profile and its assessment as an agent against oxidative stress in isolated mouse organs. Journal of Medicinal Food, 15(2), 206–215. https://doi.org/10.1089/jmf.2010.0342
Rausch, K. D., Pruiett, L. E., Wang, P., Xu, L., Belyea, R. L., & Tumbleson, M. E. (2009). Laboratory measurement of yield and composition of dry-milled corn fractions using a shortened, single-stage tempering procedure. Cereal Chemistry, 86(4), 434–438. https://doi.org/10.1094/CCHEM-86-4-0434
Rifna, E. J., Misra, N. N., & Dwivedi, M. (2021). Recent advances in extraction technologies for recovery of bioactive compounds derived from fruit and vegetable waste peels: A review. Critical Reviews in Food Science and Nutrition, 0(0), 1–34. https://doi.org/10.1080/10408398.2021.1952923
Routray, W., & Orsat, V. (2014). MAE of phenolic compounds from blueberry leaves and comparison with other extraction methods. Industrial Crops and Products, 58, 36–45. https://doi.org/10.1016/j.indcrop.2014.03.038
Roy, S., & Rhim, J. W. (2021). Anthocyanin food colorant and its application in pH-responsive color change indicator films. Critical Reviews in Food Science and Nutrition, 61(14), 2297–2325.
Simić, V. M., Rajković, K. M., Stojičević, S. S., Veličković, D. T., Nikolić, N., Lazić, M. L., & Karabegović, I. T. (2016). Optimization of microwave-assisted extraction of total polyphenolic compounds from chokeberries by response surface methodology and artificial neural network. Separation and Purification Technology, 160, 89–97. https://doi.org/10.1016/j.seppur.2016.01.019
Singh, P., Bilyeu, L., & Krishnaswamy, K. (2022). Spray drying process optimization: Drought resistant variety (W82) soymilk powder using response surface methodology (RSM). LWT - Food Science and Technology, 113760. https://doi.org/10.1016/j.lwt.2022.113760
Somavat, P., Kumar, D., & Singh, V. (2018). Techno-economic feasibility analysis of blue and purple corn processing for anthocyanin extraction and ethanol production using modified dry grind process. Industrial Crops and Products, 115(November 2017), 78–87. https://doi.org/10.1016/j.indcrop.2018.02.015
Tayal, M., Somavat, P., Rodriguez, I., Thomas, T., Christoffersen, B., & 1, R. K. (2020). Polyphenol-Rich Purple Corn Pericarp Extract Adversely Impacts Herbivore Growth and Development. Insects. http://tailieudientu.lrc.tnu.edu.vn/Upload/Collection/brief/brief_49491_54583_TN201500606.pdf
Venter, A., Fisher, H., Stafford, G. I., & Duodu, K. G. (2022). Pigmented flower extracts of plant species from the Geraniaceae and Lamiaceae families as natural food colourants: anthocyanin composition, thermal and oxidative stability. International Journal of Food Science and Technology, 4347–4355. https://doi.org/10.1111/ijfs.15761
Wen, L., Zhang, Z., Sun, D. W., Sivagnanam, S. P., & Tiwari, B. K. (2020). Combination of emerging technologies for the extraction of bioactive compounds. Critical Reviews in Food Science and Nutrition, 60(11), 1826–1841. https://doi.org/10.1080/10408398.2019.1602823
Weremfo, A., Adulley, F., & Adarkwah-Yiadom, M. (2020). Simultaneous Optimization of Microwave-Assisted Extraction of Phenolic Compounds and Antioxidant Activity of Avocado (Persea americana Mill.) Seeds Using Response Surface Methodology. Journal of Analytical Methods in Chemistry, 2020. https://doi.org/10.1155/2020/7541927
Yang, Z., & Zhai, W. (2010a). Optimization of microwave-assisted extraction of anthocyanins from purple corn (Zea mays L.) cob and identification with HPLC-MS. Innovative Food Science and Emerging Technologies, 11(3), 470–476. https://doi.org/10.1016/j.ifset.2010.03.003
Yang, Z., & Zhai, W. (2010b). Optimization of microwave-assisted extraction of anthocyanins from purple corn (Zea mays L.) cob and identification with HPLC-MS. Innovative Food Science and Emerging Technologies, 11(3), 470–476. https://doi.org/10.1016/j.ifset.2010.03.003
Zhang, W., Shen, Y., Li, Z., Xie, X., Gong, E. S., Tian, J., Si, X., Wang, Y., Gao, N., Shu, C., Meng, X., Li, B., & Liu, R. H. (2021). Effects of high hydrostatic pressure and thermal processing on anthocyanin content, polyphenol oxidase and β-glucosidase activities, color, and antioxidant activities of blueberry (Vaccinium Spp.) puree. Food Chemistry, 342(October 2020). https://doi.org/10.1016/j.foodchem.2020.128564
Zhang, Y., Tang, N., Shi, L., Miao, Y., Liu, X., Ge, X., Cheng, Y., & Zhang, X. (2020). Characterization and comparison of predominant aroma compounds in microwave-treated wheat germ and evaluation of microwave radiation on stability. Journal of Cereal Science, 93(February), 102942. https://doi.org/10.1016/j.jcs.2020.102942
Zhao, M., Luo, Y., Li, Y., Liu, X., Wua, J., Liao, X., & Chen, F. (2013). The identification of degradation products and degradation pathway of malvidin-3-glucoside and malvidin-3,5-diglucoside under microwave treatment. Food Chemistry, 141(3), 3260–3267. https://doi.org/10.1016/j.foodchem.2013.05.147
Zhou, G., Ma, J., Tang, Y., Wang, X., Zhang, J., Yao, X., Jiang, W., & Duan, J. A. (2018). Optimization of Ultrasound-Assisted Extraction Followed by Macroporous Resin Purification for Maximal Recovery of Functional Components and Removal of Toxic Components from Ginkgo biloba Leaves. BioMed Research International, 2018. https://doi.org/10.1155/2018/4598067
Zinoviadou, K. G., Galanakis, C. M., Brnčić, M., Grimi, N., Boussetta, N., Mota, M. J., Saraiva, J. A., Patras, A., Tiwari, B., & Barba, F. J. (2015). Fruit juice sonication: Implications on food safety and physicochemical and nutritional properties. Food Research International, 77, 743–752. https://doi.org/10.1016/j.foodres.2015.05.032
Zou, M., Zhang, W., Wu, R., Jiang, H., Cao, F., & Su, E. (2021). Removal of ginkgotoxin from the Ginkgo biloba seeds powder by adopting membrane separation technology. Journal of Cleaner Production, 280, 124452. https://doi.org/10.1016/j.jclepro.2020.124452
Acknowledgements
The authors acknowledge Prof. Mengshi Lin and Prof. Bongkosh Vardhanabhuti for their help with the FTIR and Zetasizer equipment.
Funding
This research was funded by the USDA NIFA Research Award No. 2022–69016-36101.
Author information
Authors and Affiliations
Contributions
Isaac Duah Boateng; Investigation, Methodology, Data curation, Software, Formal analysis, Writing the entire manuscript, and Validation. Azlin Mustapha; Supervision, Validation, Review & editing. Christopher R. Daubert; Review & editing, Validation. Lucas Kuehnel; Data curation, Ravinder Kumar; Data curation, Joseph Agliata: Data curation, Caixia Wan; Review & editing. Sherry Flint-Garcia; Review & editing. Pavel Somavat; Conceptualization, Funding acquisition, Supervision, review & editing.
Corresponding author
Ethics declarations
Competing Interest
No conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boateng, I.D., Mustapha, A., Daubert, C.R. et al. Novel Two-pot Microwave Extraction of Purple Corn Pericarp’s Phenolics and Evaluation of the Polyphenol-rich Extract’s Product Quality, Bioactivities, and Structural Properties. Food Bioprocess Technol 16, 2668–2691 (2023). https://doi.org/10.1007/s11947-023-03072-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-023-03072-7