Abstract
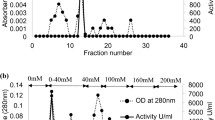
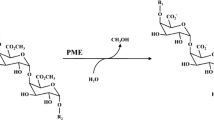
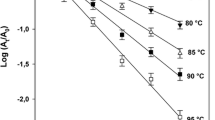
Pectin methylesterases (PMEs) from two different orange sources, Valencia and Navel cv., were extracted and purified using cation exchange, heparin chromatography, and finally, gel filtration chromatography, yielding a single peak corresponding to a protein of molecular weight 34 and 35 kDa for Valencia and Navel PMEs, respectively. Effects of high pressure (HP) and thermal processing for various treatment durations on the activity of PMEs in Tris–HCl buffer solution (pH 7.5) were explored. Higher levels of pressure, temperature, and treatment duration resulted in an analogous reduction of the PME residual activity. HP-induced inactivation of both purified PMEs was described by a first-order kinetic model. Kinetic parameters were estimated and a multiparameter equation was developed to predict the PME inactivation rate constant at any combination of pressure and temperature conditions for both enzymes. The PME from Valencia orange peel appeared to be more heat- and pressure-sensitive compared to Navel PME. HP-induced conformational changes of the PME molecules were also investigated using circular dichroism (CD) spectroscopy. A direct comparison of the CD results for treated and untreated proteins reveals that pressure treatment has negligible effects upon far-UV CD spectra, while significant irreversible changes are depicted in near UV for both PMEs. It is, thus, evidenced that exposure to HP may lead to a structurally molten globulelike state, where the PME maintains a secondary structure of untreated protein molecules, while a tertiary structure is substantially affected bearing subsequent impact on substrate–enzyme binding interaction, leading to reduction of enzyme activity.






Similar content being viewed by others
References
Balan, A., Santa-Cruz, C. P., Moutran, A., Ferreira, R. C. C., Medrano, F. J., Pérez, C. A., et al. (2006). The molybdate-binding protein (ModA) of the phytopathogen Xanthomonas axonopodis pv citri. Protein Expression and Purification, 50(2), 215–222.
Balogh, T., Smout, C., Ly Nguyen, B., Van Loey, A., & Hendrickx, M. (2004). Thermal and high pressure inactivation kinetics of carrot pectinmethylesterase (PME): From model systems to real foods. Innovative Food Science & Emerging Technologies, 5(4), 429–436.
Barbosa-Cánovas, G. V. (1999). Preservation of foods with pulsed electric fields. London: Academic.
Böhm, G., Muhr, R., & Jaenicke, R. (1992). Quantitative analysis of protein far UV circular dichroism spectra by neural networks. Protein Engineering, 5(3), 191–195.
Boulekou, S., Katsaros, G., & Taoukis, P. (2010). Inactivation kinetics of peach pulp pectin methylesterase as a function of high hydrostatic pressure and temperature process conditions. Food and Bioprocess Technology, 3(5), 699–706.
Bradford, M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry, 72, 248–254.
Cameron, R. G., Baker, R. A., & Grohmann, K. (1996). Citrus tissue extracts affect juice cloud stability. Journal of Food Science, 62, 242–245.
Cameron, G. R., Savary, B. J., Hotchkiss, A. T., & Fishman, M. L. (2005). Isolation, characterization and pectin-modifying properties of a thermally tolerant pectin methylesterase from Citrus sinensis var. Valencia. Journal of Agricultural and Food Chemistry, 53(6), 2255–2260.
Castro, S., Saraiva, J., Lopes-da-Silva, J., Delgadillo, I., Van Loey, A., Smout, C., et al. (2008). Effect of thermal blanching and of high pressure treatments on sweet green and red bell pepper fruits (Capsicum annuum L.). Food Chemistry, 107(4), 1436–1449.
Castro, A. J., Barbosa-Cánovas, G. V., & Swanson, B. G. (1993). Microbial inactivation of foods by pulsed electric fields. Journal of Food Processing and Preservation, 17(1), 47–73.
Chang, C. T., Wu, C.-S. C., & Yang, J. T. (1978). Circular dichroic analysis of protein conformation: Inclusion of the β-turns. Analytical Biochemistry, 91(1), 13–31.
Eagerman, B. A., & Rouse, A. H. (1976). Heat inactivation temperature–time relationships for pectin esterase inactivation in citrus juices. Journal of Food Science, 41(6), 1396–1397.
Foguel, D., & Silva, J. L. (1994). Cold denaturation of a repressor–operator complex: The role of entropy in protein-DNA recognition. Proceedings of the National Academy of Sciences, USA, 91(17), 8244–8247.
Gervilla, R., Ferragut, V., & Guamis, B. (2000). High pressure inactivation of microorganisms inoculated into ovine milk of different fat contents. Journal of Dairy Science, 83(4), 674–682.
Goto, Y., & Fink, A. L. (1989). Conformational states of beta-lactamase: Molten-globule states at acidic and alkaline pH with high salt. Biochemistry, 28(3), 945–952.
Guiavarc'h, Y., Segovia, O., Hendrickx, M., & Van Loey, A. (2005). Purification, characterization, thermal and high-pressure inactivation of a pectin methylesterase from white grapefruit (Citrus paradisi). Innovative Food Science and Emerging Technologies, 6(4), 363–371.
Hendrickx, M., Ludikhuyze, L., Van den Broeck, I., & Weemaes, C. (1998). Effects of high pressure on enzymes related to food quality.Trends of. Food Science and Technology, 9(5), 107–203.
Hendrickx M & Knorr D (2002) Ultra high pressure treatment of foods. Kluwer, New York, Aspen food engineering series.
Heremans, K. (1982). High pressure effects on proteins and other biomolecules. Annual Review of Biophysics and Bioengineering, 11, 1–21.
Hummer, G., Garde, S., Garcia, A. E., Paulaitis, M. E., & Pratt, L. R. (1998). The pressure dependence of hydrophobic interactions is consistent with the observed pressure denaturation of proteins. Proceedings of the National Academy of Sciences of the United States of America, 95(4), 1552–1555.
Johansson, K., El Ahmad, M., Friemann, R., Jornvall, H., Markovic, O., & Eklund, H. (2002). Crystal structure of plant pectin methylesterase. FEBS Letters, 514(2–3), 243–249.
Katsaros, G., Katapodis, P., & Taoukis, P. (2009a). High hydrostatic pressure inactivation kinetics of the plant proteases ficin and papain. Journal of Food Engineering, 91(1), 42–48.
Katsaros, G., Giannoglou, M., & Taoukis, P. (2009b). Kinetic study of the combined effect of high hydrostatic pressure and temperature on the activity of Lactobacillus delbrueckii ssp. bulgaricus aminopeptidases. Journal of Food Science, 74(5), 219–225.
Katsaros, G., Tsevdou, M., Panagiotou, T., & Taoukis, P. (2010a). Kinetic study of high pressure microbial and enzyme inactivation and selection of pasteurization conditions. International Journal of Food Science and Technology, 45(6), 1119–1129.
Katsaros, G., Tavantzis, G., & Taoukis, P. (2010b). Production of novel dairy products using actinidin and high pressure as enzyme activity regulator. Innovative Food Science and Emerging Technologies, 11(1), 47–51.
Laratta, B., Masi, L. D., Minasi, P., & Giovane, A. (2008). Pectin methylesterase in Citrus bergamia R. Purification, biochemical characterization and sequence of the exon related to the enzyme active site. Food Chemistry, 110(4), 829–837.
Ly-Nguyen, B., Van Loey, A. M., Smout, C., ErenOzcan, S., Fachin, D., Verlent, I., & Hendrickx, M. E. (2003a). Mild heat and high-pressure inactivation of carrot pectinmethylesterase: A kinetic study. Journal of Food Science, 68(4), 1377–1383.
Ly-Nguyen, B., Loey, A. M. V., Smout, C., Verlent, I., Duvetter, T., & Hendrickx, M. E. (2003b). Effect of mild-heat and high-pressure processing on banana pectin methylesterase: A kinetic study. Journal of Agricultural and Food Chemistry, 51(27), 7974–7979.
Menéndez, O. H., Schwarzenbolz, U. R., & Henle, T. (2006). Structural changes of microbial transglutaminase during thermal and high-pressure treatment. Journal of Agricultural and Food Chemistry, 54(5), 1716–1721.
Messens, W., Camp, J. V., & Huyghebaert, A. (1997). The use of high pressure to modify the functionality of food proteins. Trends in Food Science and Technology, 8(4), 107–112.
Mozhaev, V., Heremans, K., Frank, J., Masson, P., & Balny, C. (1994). Exploiting the effects of high hydrostatic pressure in biotechnological applications. Trends in Biotechnology, 12(12), 493–501.
Mozhaev, V., Heremans, K., Frank, J., Masson, P., & Balny, C. (1996). High pressure effects on protein structure and function. Proteins: Structure,Function and Bionformatics, 24(1), 81–91.
Nienaber, U., & Shellhammer, T. H. (2001). High-pressure processing of orange juice: Kinetics of pectinmethylesterase inactivation. Journal of Food Science, 66(2), 328–331.
Polydera, A., Galanou, E., Stoforos, N., & Taoukis, P. (2004). Inactivation kinetics of pectin methylesterase of Greek Navel orange juice as a function of high hydrostatic pressure and temperature process conditions. Journal of Food Engineering, 62(3), 291–298.
Ramos, C. H. I. (2004). A spectroscopic-based laboratory course for protein conformational studies. Biochemistry and Molecular Biology Education, 32(1), 31–34.
Ramos, C. H. I., Kay, M. S., & Baldwin, R. L. (1999). Putative interhelix ion pairs involved in the stability of myoglobin. Biochemistry, 38(30), 9783–9790.
Ramos, C. H. I., Weisbuch, S., & Jamin, M. (2007). Diffusive motions control the folding and unfolding kinetics of apomyoglobin pH 4 molten globule intermediate. Biochemistry, 46(14), 4379–4389.
Ravindra, R., & Winter, R. (2003). On the temperature-pressure free-energy landscape of proteins. Chem PhysChem, 4(4), 359–365.
Regis, W. C. B., Fattori, J., Santoro, M. M., Jamin, M., & Ramos, C. H. I. (2005). On the difference in stability between horse and sperm whale myoglobins. Archives of Biochemistry and Biophysics, 436(1), 168–177.
Ribeiro-Jr, E. A., & Ramos, C. H. I. (2004). Origin of the anomalous circular dichroism spectra of many apomyoglobin mutants. Analytical Biochemistry, 329(2), 300–306.
Rombouts, F. M., Versteeg, C., Karman, A. H., & Pilnik, W. (1982). Pectinesterases in component parts of citrus fruits related to problems of cloud loss and gelation in citrus products. In P. Dupuy (Ed.), Use of enzymes in food technology (pp. 483–487). Paris, France: Technique et documentation Lavoisier.
Rouse, A. H., & Atkins, C. D. (1955). Pectinesterase and pectin in commercial orange juice as determined by methods used at the Citrus Experiment Station. Bulletin of the University of Florida Agricultural Experiment Station, 570, 1–19.
Sampedro, F., Rodrigo, D., & Hendrickx, M. (2008). Inactivation kinetics of pectin methyl esterase under combined thermal–high pressure treatment in an orange juice–milk beverage. Journal of Food Engineering, 86(1), 133–139.
Savary, B. J., Vasu, P., Nuñez, A., & Cameron, R. G. (2010). Identification of thermolabile pectin methylesterases from sweet orange fruit by peptide mass fingerprinting. Journal of Agricultural and Food Chemistry, 58, 12462–12468.
Sila, D., Smout, C., Satara, Y., Truong, V., Van Loey, A., & Hendrickx, M. (2007). Combined thermal and high pressure effect on carrot pectinmethylesterase stability and catalytic activity. Journal of Food Engineering, 78(3), 755–764.
Tedford, L. A., Smith, D., & Schaschke, C. J. (1999). High pressure processing effects on the molecular structure of ovalbumin, lysozyme and β-lactoglobulin. Food Research International, 32(2), 101–106.
Thanassoulas, A., Nomikos, M., Theodoridou, M., Stavros, P., Mastellos, D., & Nounesis, G. (2011). Thermal and chemical denaturation of the BRCT functional module of human 53BP1. International Journal of Biological Macromolecules, 49(3), 297–304.
Tiwari, B. K., Muthukumarappan, K., O'Donnell, C. P., & Cullen, P. J. (2009). Inactivation kinetics of pectin methylesterase and cloud retention in sonicated orange juice. Innovative Food Science and Emerging Technologies, 10(2), 166–171.
Van den Broeck, I., Ludikhuyze, L. R., Van Loey, A. M., & Hendrickx, M. (2000a). Effect of temperature and/or pressure on tomato pectinesterase activity. Journal of Agricultural and Food Chemistry, 48(2), 551–558.
Van den Broeck, I., Ludikhuyze, L. R., Van Loey, A. M., & Hendrickx, M. E. (2000b). Inactivation of orange pectinesterase by combined high-pressure and -temperature treatments: A kinetic study. Journal of Agricultural and Food Chemistry, 48(5), 1960–1970.
Versteeg, C., Rombouts, F. M., & Pilnik, W. (1978). Purification and some characteristics of two pectinesterase isoenzymes from orange. Lebensmittel-Wissenschaft und Technologie, 11, 267–274.
Vidugiris, G. J. A., & Royer, C. A. (1998). Determination of the volume changes for the pressure-induced transitions of apomyoglobin between the native, molten globule and unfolded states. Biophysical Journal, 75(1), 463–470.
Woody, R. W. (1995). Circular dichroism. Methods in Enzymology, 246, 34–71.
Zhong, K., Wu, J., Wang, Z., Chen, F., Liao, X., & Hu, X. (2007). Inactivation kinetics and secondary structural change of PEF treated POD and PPO. Food Chemistry, 100(1), 115–123.
Zhou, L., Wu, J., Hu, X., Zhi, X., & Liao, X. (2009). Alterations in the activity and structure of pectin methylesterase treated by high pressure carbon dioxide. Journal of Agricultural and Food Chemistry, 57(5), 1890–1895.
Acknowledgments
The authors would like to thank Professor P. Christakopoulos and Lecturer E. Topakas for offering their expert advice and experience on enzyme purification assessment. This research has been cofinanced by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program: Heracleitus II. Investing in knowledge society through the European Social Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alexandrakis, Z., Katsaros, G., Stavros, P. et al. Comparative Structural Changes and Inactivation Kinetics of Pectin Methylesterases from Different Orange Cultivars Processed by High Pressure. Food Bioprocess Technol 7, 853–867 (2014). https://doi.org/10.1007/s11947-013-1087-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-013-1087-7