Abstract
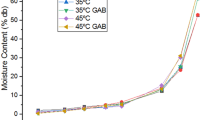
The moisture adsorption isotherms of solar dehydrated mango and jackfruit were determined at temperatures ranging from 30 °C to 50 °C. The equilibrium moisture content (EMC) of mango and jackfruit increased sharply as the temperature increased at water activity (a w) above 0.6 and 0.8, respectively. However, there were no clear isothermal intersection points observed at higher a w and temperatures. The EMC of solar dehydrated jackfruit showed the isothermal characteristics between types II and III. In contrast, dehydrated mango followed the characteristic type III adsorption isotherms due to high total soluble solids content of 67.8 °Brix and total sugars of 14.21 g/100 g fresh mango. Estimated parameters and fitting ability of three isotherm models were also evaluated. The Guggenheim-Anderson-Boer (GAB) model gave the best fit to the experimental EMC data. The GAB monolayer moisture contents (m o) of mango and jackfruit ranged from 11.1–10.0 % and 4.7–3.4 %, respectively. Specific surface area of active binding sites (S) was calculated based on the m o values. The S value of dehydrated mango was 2.5 to 2.8 times larger than jackfruit. The maximum net isosteric heat (q s) of sorption of solar dehydrated mango and jackfruit were determined as 19.5 and 33 kJ mol−1, respectively, and q s decreased significantly at high moisture.



Similar content being viewed by others
Abbreviations
- a, b, c :
-
Henderson and Chung-Pfost model parameters
- a w :
-
Water activity
- C K :
-
GAB model parameters
- c o k o :
-
Entropic accommodation factors
- d.b.:
-
Dry basis (%)
- df:
-
Degrees of freedom
- e :
-
Temperature dependence constant of monolayer (K)
- EMC:
-
Equilibrium moisture content (g/100 g dry matter)
- ERH:
-
Equilibrium relative humidity (%)
- g :
-
Monolayer and multiplayer water enthalpy difference (K)
- GAB:
-
Guggenheim-Anderson-Boer equations
- h i :
-
Molar sorption enthalpy of free water (J mol−1)
- h m :
-
Monolayer sorption enthalpy (J mol−1)
- h n :
-
Multilayer molar sorption enthalpy (J mol−1)
- i :
-
Multilayer and free water enthalpy difference (K)
- m :
-
Moisture content (g/100 g dry matter)
- M :
-
Moisture content (% d.b.)
- m a :
-
Temperature dependence of monolayer (K)
- MCPE:
-
Modified Chung-Pfost equation
- MHEE:
-
Modified Henderson equation
- m i :
-
Observed moisture content (g/100 g dry matter)
- m o :
-
Monolayer moisture content (g/100 g dry matter)
- m p :
-
Predicted moisture content (g/100 g dry matter)
- P :
-
Mean relative percentage deviation modulus (%)
- p :
-
Probability value
- q s :
-
Isosteric heat of sorption (J mol−1)
- R :
-
Universal gas constant (8.314 J mol−1 K−1)
- r.h.:
-
Relative humidity (%)
- R 2 :
-
Regression coefficient
- rpm:
-
Round per minute
- RSS:
-
Residual sum of squares
- S :
-
Specific surface area (m2 g−1)
- SD:
-
Standard deviation
- SEE:
-
Standard error of estimate
- T :
-
Absolute temperature (K)
- TA:
-
Titratable acidity (%)
- TSS:
-
Total soluble solids content (°Brix)
- w.b.:
-
Wet basis (%)
References
Aguerre, R. J., Suarez, C., & Viollaz, P. E. (1989). Swelling and pore structure in starchy materials. Journal of Food Engineering, 9, 71–80.
AOAC. (1996). Official method of analysis. Washington, DC: Association of Official Analytical Chemists.
Bellagha, S., Sahli, A., & Farhat, A. (2008). Desorption isotherms and isoseteric heat of three Tunisian date cultivars. Food and Bioprocess Technology, 1, 270–275.
Blahovec, J. (2004). Sorption isotherms in materials of biological origin mathematical and physical approach. Journal of Food Engineering, 65, 489–495.
Blahovec, J., & Yanniotis, S. (2008). GAB generalized equation for sorption phenomena. Food and Bioprocess Technology, 1, 82–90.
Cernisev, S. (2010). Effect of conventional and multistage drying processing on non-enzymatic browning in tomato. Journal of Food Engineering, 96, 114–118.
Chowdhury, F. A., Raman, M. A., & Mian, A. J. (1997). Distribution of free sugars and fatty acids in jackfruit (Artocarpus heterophyllus). Food Chemistry, 60, 25–28.
Chung, S. G., & Pfost, H. B. (1976). Summarizing and reporting equilibrium moisture data for grains (pp. 76–3520). St. Joseph: American Society of Agriculture Engineers.
Esper, A., & Mühlbauer, W. (1996). Solar tunnel dryer for fruits. Plant Research and Development, 44, 61–80.
Falade, K. O., Olukini, I., & Adegoke, G. O. (2004). Adsorption isotherm and heat of sorption of somatically pretreated and air-dried pineapples slices. European Food Research and Technology, 218, 540–543.
Hayoglu, I., & Gamli, O. F. (2007). Water sorption isotherms of pistachio nut paste. International Journal of Food Science and Technology, 43, 224–227.
Hubinger, M., Menegalli, F. C., Aguerre, R. J., & Suarez, C. (1992). Water vapor adsorption isotherms of guava, mango and pineapple. Journal of Food Science, 57, 1405–1407.
Illeperuma, C. K., & Jayasuriya, P. (2002). Prolonged storage of ‘Karthakolomban’ mango by modified atmosphere packaging at low temperature. The Journal of Horticultural Science and Biotechnology, 77, 153–157.
SAS Institute (1990) SAS language and procedures, version 6.1st edition. SAS Institute, Cary, NC.
Isse, M. G., Schuchmann, H., & Schubert, H. (1993). Divided sorption isotherm concept: An alternative way to describe sorption isotherm data. Journal of Food Process Engineering, 16, 147–157.
Kiranoudis, C. T., Maroulis, Z. B., Tsami, M., & Marinos-Kouris, D. (1993). Equilibrium moisture content and heat of desorption of some vegetables. Journal of Food Engineering, 20, 55–74.
Labuza, T. P., Kannane, A., & Chen, J. (1985). Effect of temperature on the moisture sorption isotherm and water activity shift of two dehydrated foods. Journal of Food Science, 50, 385–392.
Lewicki, P. P. (1997). The applicability of the GAB model to food water sorption isotherms. International Journal of Food Science and Technology, 32, 553–557.
Lutz, K., Muhlbauer, W., Muller, J., & Reisinger, G. (1987). Development of multi-purpose solar crop dryer for arid zones. Solar and Wind Technology, 4, 417–428.
Mir, A. M., & Nath, N. (1995). Sorption isotherms of fortified mango bars. Journal of Food Engineering, 25, 141–150.
Ngoddy, P. O., & Bakker-Arkema, F. W. (1970). A generalized theory of sorption phenomena in biological materials (Part I. The isotherm equation). Transactions of ASAE, 13, 612–617.
Ngoddy, P. O., & Bakker-Arkema, F. W. (1972). A generalized theory of sorption phenomena in biological materials. Part III—A pore size distribution function of the power law type for biological materials. Transactions of ASAE, 15, 1160–1164.
Quirijns, E. J., van Boxtel, A. J. B., van Loon, W. K. P., & van Straten, G. (2005). Sorption isotherms, GAB parameters and isosteric heat of sorption. Journal of the Science of Food and Agriculture, 85, 1805–1814.
Rahman, A. K. M., Huq, E., Mian, A. J., & Chesson, A. (1995). Microscopic and chemical changes occurring during the ripening of two forms of jackfruit (Artocarpus heterophyllus L.). Food Chemistry, 52, 405–410.
Ranganna, S. (1986). Hand book of analysis and quality control for fruit and vegetable products (2nd ed.). New Delhi: Tata McGraw-Hill Publishing Company Limited.
Saravacos, G. D., Tsiourvas, D. A., & Tsami, E. (1986). Effect of temperature on the water adsorption isotherms of sultana raisins. Journal of Food Science, 51, 381–383.
Sato, Y., Wada, Y., & Higo, A. (2010). Relationship between monolayer and multilayer water contents, and involvement in gelatinization of baked starch products. Journal of Food Engineering, 96, 172–178.
Schirmer, P., Janjai, S., Esper, A., Smitabhindu, R., & Muhlbauer, W. (1996). Experimental investment of the performance of the solar tunnel dryer for drying bananas. Renewable Energy, 7, 119–129.
Singh, P. C., & Singh, R. K. (1996). Application of GAB model for water sorption isotherms of food products. Journal of Food Processing and Preservation, 20, 203–220.
Singh, B., Panesar, P. S., Gupta, A. K., & Kennedy, J. F. (2006). Sorption isotherm behavior of osmoconvectively dehydrated carrot cubes. Journal of Food Processing and Preservation, 30, 684–698.
Sun, D.-W. (1999). Comparison and selection of EMC/ERH isotherm equations for rice. Journal of Stored Products Research, 35, 249–264.
Sun, D.-W., & Woods, J. L. (1994). The selection of sorption isotherm equation for wheat based on the fitting of available data. Journal of Stored Products Research, 24, 22–474.
Thompson, T. L., Peart, R. M., & Foster, G. H. (1968). Mathematical simulation of corn drying—A new model. Transactions of ASAE, 11, 582–586.
Togrul, H., & Arslan, N. (2006). Moisture sorption behaviour and thermodynamic characteristics of rice stored in a chamber under controlled humidity. Biosystems Engineering, 95, 181–195.
Tortoe, C., & Orchard, J. (2006). Microstructural changes of osmotically dehydrated tissues of apple, banana and potato. Scanning, 28, 172–178.
Tsami, E., Marinos-Kouris, D., & Maroulis, Z. B. (1990). Water sorption isotherms of raisins, currants, figs, prunes and apricots. Journal of Food Science, 55, 1594–1597.
Tsami, E., Maroulis, Z. B., Marinos-Kouris, D., & Saravacos, G. D. (1990). Heat of sorption of water in dried fruits. International Journal of Food Science and Technology, 25, 350–359.
Van den Berg, C. (1984). Description of water activity of food for engineering purposes by means of the GAB model of sorption. In B. M. Mckenna (Ed.), Engineering and Food (pp. 21–311). London: Elsevier Applied Science.
Verma, V. K., & Narain, M. (1990). Moisture absorption isotherms of jaggery. Journal of Stored Product Research, 26, 61–66.
Wexler, A. (1999). Constant humidity solutions. In D. R. Lide (Ed.), CRC handbook of chemistry and physics (pp. 15–25). New York: CRC Press.
Yanniotis, S., & Blahovec, J. (2009). Model analysis of sorption isotherm. LWT-Food Science and Technology, 42, 1688–1695.
Acknowledgment
We thank Jatal Mannapperuma (PGIA, University of Peradeniya, Sri Lanka) and Judy Johnson (USDA-ARS, Parlier, CA 93648, USA) for their valuable guidance and support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prasantha, B.D.R., Amunogoda, P.N.R.J. Moisture Adsorption Characteristics of Solar-Dehydrated Mango and Jackfruit. Food Bioprocess Technol 6, 1720–1728 (2013). https://doi.org/10.1007/s11947-012-0832-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-012-0832-7