Abstract
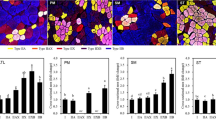
Changes of meat shear force and its characteristics during cooking have been extensively studied, but great variability existed due to the cooking method among different studies. This study was designed to focus on the dynamic changes of beef intramuscular connective tissue (IMCT) and muscle fiber during water-bath heating and their effects on beef shear force. At 4 d postmortem, beef semitendinosus muscles were divided into 11 steaks and then cooked respectively to an internal temperature of 40, 50, 55, 60, 65, 70, 75, 80, 85, and 90°C (the remainder was not cooked as control). Collagen content and its solubility, transition temperature of perimysia and endomysia, fiber diameter, and Warner–Bratzler shear force values (WBSF) were determined. The results showed that fiber diameter decreased gradually during cooking, concomitant with the increases in filtering residue and WBSF. The maximum transition temperature (T max) of endomysial components was lower than that of perimysial components (50.2 vs. 65.2°C). Muscle fiber and IMCT (especially perimysia) shrank during cooking, resulting in the increase of WBSF when the internal temperature was lower than 75°C, but further cooking led to the disintegration of perimysial structure, lowing up the increase of WBSF between 75 and 90°C. For beef semitendinosus muscle, the internal temperature of 65°C is a critical cooking point where meat gets tougher.



Similar content being viewed by others
References
American Meat Science Association (1995). Research guidelines for cookery, sensory evaluation and instrumental tenderness measurements of fresh meat. Chicago: National Live Stock and Meat Board.
Bergman, I., & Loxley, R. (1963). Two improved and simplified methods for spectrophotometric determination of hydroxyproline. Analytical Chemistry, 35, 1961–1965. doi:10.1021/ac60205a053.
Bouton, P. E., & Harris, P. V. (1972). The effects of some postslaughter treatments on the mechanical properties of bovine and ovine muscle. Journal of Food Science, 37, 539–543. doi:10.1111/j.1365-2621.1972.tb02687.x.
Bouton, P. E., Harris, P. V., & Ratcliff, D. (1981). Effect of cooking temperature and time on the shear properties of meat. Journal of Food Science, 46, 1082–1087. doi:10.1111/j.1365-2621.1981.tb02996.x.
Christensen, M., Purslow, P. P., & Larsen, L. M. (2000). The effect of cooking temperature on mechanical properties of whole meat, single muscle fibers and perimysial connective tissue. Meat Science, 55, 301–307. doi:10.1016/S0309-1740(99)00157-6.
Davey, C. L., & Gilbert, K. V. (1974). Temperature dependent cooking toughness in beef. Journal of the Science of Food and Agriculture, 25, 931–938. doi:10.1002/jsfa.2740250808.
Fang, S. H., Nishimura, T., & Takahashi, K. (1999). Relationship between development of intramuscular connective tissue and toughness of pork during growth of pigs. Journal of Animal Science, 77, 120–130.
Flint, F. O., & Pickering, K. (1984). Demonstration of collagen in meat products by an improved picro-sirius red polarization method. Analyst (Lond), 109, 1505–1508. doi:10.1039/an9840901505.
Hill, F. (1966). The solubility of intramuscular collagen in meat animals of various ages. Journal of Food Science, 31, 161–166. doi:10.1111/j.1365-2621.1966.tb00472.x.
Kopp, J., & Bonnet, M. (1987). Stress–strain and isometric tension measurements in collagen. In A. M. Pearson, T. M. Dutson, & A. J. Baily (Eds.), Advances in meat research vol. 4: Collagen as a food (pp. 163–185). New York: Van Nostrand.
Lawrie, R. A., & Ledward, D. A. (2006). Lawrie’s meat science (17th ed.). Cambridge: Woodhead Publishing.
Light, N., & Champion, A. E. (1984). Characterization of muscle epimysium, perimysium and endomysium collagens. Biochemical Journal, 219, 1017–26.
López Osornio, M. M., Hough, G., Salvador, A., Chambers IV, E., McGraw, S., & Fiszman, S. (2008). Beef’s optimum internal cooking temperature as seen by consumers from different countries using survival analysis statistics. Food Quality and Preference, 19, 12–20.
Martens, H., Stabussvik, E., & Martens, M. (1982). Texture and color changes in meat during cooking related to thermal denaturation of muscle proteins. Journal of Texture Studies, 13, 291–309. doi:10.1111/j.1745-4603.1982.tb00885.x.
Nishimura, T., Hattori, A., & Takahashi, K. (1999). Structural changes in intramuscular connective tissue during the fattening of Japanese Black cattle, effect of marbling on beef tenderization. Journal of Animal Science, 77, 93–104.
Powell, T. H., Dikeman, M. E., & Hunt, M. C. (2000). Tenderness and collagen composition of beef semitendinosus roasts cooked by conventional convective cooking and modeled, multistage convective cooking. Meat Science, 55, 421–425. doi:10.1016/S0309-1740(99)00171-0.
Purslow, P. P. (1999). The intramuscular connective tissue matrix and cell–matrix interactions in relation to meat toughness. In Proceedings of the 45th International Congress of Meat Science and Technology, Yokohama, Japan (pp. 210–219).
Torrescano, G., Sánchez, E. A., Giménez, B., Roncalés, P., & Beltrán, J. A. (2003). Shear values of raw samples of 14 bovine muscles and their relation to muscle collagen characteristics. Meat Science, 64, 85–91. doi:10.1016/S0309-1740(02)00165-1.
Wright, D. J., & Wilding, P. (1984). Differential scanning calorimetric study of muscle and its proteins: Myosin and its subfragments. Journal of the Science of Food and Agriculture, 35, 357–372. doi:10.1002/jsfa.2740350317.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, C.B., Zhou, G.H. & Xu, X.L. Dynamical Changes of Beef Intramuscular Connective Tissue and Muscle Fiber during Heating and their Effects on Beef Shear Force. Food Bioprocess Technol 3, 521–527 (2010). https://doi.org/10.1007/s11947-008-0117-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-008-0117-3