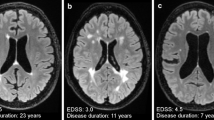
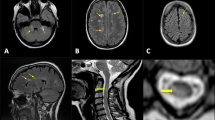
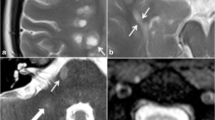
Opinion statement
Multiple sclerosis (MS) is an immune-mediated disease affecting the central nervous system (CNS). Magnetic resonance imaging (MRI) has long been recognized as an important tool in the diagnosis of MS. It is increasingly recognized that in addition to its role in diagnosis, MRI can play a key role as a noninvasive tool for prognostication, disease monitoring, assessment of treatment efficacy, and safety monitoring of disease-modifying therapies (DMTs). A confluence of factors, including increased availability of MRI, development of improved MRI techniques, and increased availability of DMTs have contributed to the expanding role of MRI in MS clinical care. As the clinical use of MRI in MS expands, it is important that MRI protocols amongst clinical centers are standardized. Here, we summarize recent evidence supporting the use of MRI in clinical practice, summarize various clinical guidelines and recommendations for the use of MRI in MS disease monitoring, and provide our recommendations for standardized MRI protocols.


Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121–7.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi:10.1002/ana.22366.
Erbayat Altay E, Fisher E, Jones SE, Hara-Cleaver C, Lee JC, Rudick RA. Reliability of classifying multiple sclerosis disease activity using magnetic resonance imaging in a multiple sclerosis clinic. JAMA Neurol. 2013;70(3):338–44. doi:10.1001/2013.jamaneurol.211.
Traboulsee A, Letourneau-Guillon L, Freedman MS, O’Connor PW, Bharatha A, Chakraborty S, et al. Canadian expert panel recommendations for MRI use in MS diagnosis and monitoring. Can J Neurol Sci. 2015;42(3):159–67. doi:10.1017/cjn.2015.24.
• Rovira A, Wattjes MP, Tintore M, Tur C, Yousry TA, Sormani MP, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis—clinical implementation in the diagnostic process. Nat Rev Neurol. 2015;11(8):471–82. doi:10.1038/nrneurol.2015.106. These guidelines provide an up-to-date, evidence-based approach on the use of MRI in the diagnosis and management of patients with multiple sclerosis.
Okuda DT, Mowry EM, Beheshtian A, Waubant E, Baranzini SE, Goodin DS, et al. Incidental MRI anomalies suggestive of multiple sclerosis: the radiologically isolated syndrome. Neurology. 2009;72(9):800–5. doi:10.1212/01.wnl.0000335764.14513.1a.
Granberg T, Martola J, Kristoffersen-Wiberg M, Aspelin P, Fredrikson S. Radiologically isolated syndrome—incidental magnetic resonance imaging findings suggestive of multiple sclerosis, a systematic review. Mult Scler. 2013;19(3):271–80. doi:10.1177/1352458512451943.
Barkhof F, Filippi M, Miller DH, Scheltens P, Campi A, Polman CH, et al. Comparison of MRI criteria at first presentation to predict conversion to clinically definite multiple sclerosis. Brain. 1997;120(Pt 11):2059–69.
•• Traboulsee A, Simon JH, Stone L, Fisher E, Jones DE, Malhotra A, et al. Revised recommendations of the consortium of MS centers task force for a standardized MRI protocol and clinical guidelines for the diagnosis and follow-up of multiple sclerosis. AJNR Am J Neuroradiol. 2016;37(3):394–401. doi:10.3174/ajnr.A4539. This review provides the latest recommendations on standardized MRI protocols for use in the clincial management of multiple sclerosis.
Wattjes MP, Barkhof F. High field MRI in the diagnosis of multiple sclerosis: high field-high yield? Neuroradiology. 2009;51(5):279–92. doi:10.1007/s00234-009-0512-0.
Patzig M, Burke M, Bruckmann H, Fesl G. Comparison of 3D cube FLAIR with 2D FLAIR for multiple sclerosis imaging at 3 Tesla. RoFo : Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2014;186(5):484–8. doi:10.1055/s-0033-1355896.
Gramsch C, Nensa F, Kastrup O, Maderwald S, Deuschl C, Ringelstein A, et al. Diagnostic value of 3D fluid attenuated inversion recovery sequence in multiple sclerosis. Acta Radiol. 2015;56(5):622–7. doi:10.1177/0284185114534413.
• Yousry TA, Pelletier D, Cadavid D, Gass A, Richert ND, Radue EW, et al. Magnetic resonance imaging pattern in natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2012;72(5):779–87. doi:10.1002/ana.23676. This study highlights characteristic MRI findings of progressive multifocal leukoencephalopathy in natalizumab-treated patients with multiple sclerosis.
Abdoli M, Chakraborty S, MacLean HJ, Freedman MS. The evaluation of MRI diffusion values of active demyelinating lesions in multiple sclerosis. Mult Scler Relat Disord. 2016;10:97–102. doi:10.1016/j.msard.2016.09.006.
Wattjes MP, Steenwijk MD, Stangel M. MRI in the diagnosis and monitoring of multiple sclerosis: an update. Clin Neuroradiol. 2015;25(Suppl 2):157–65. doi:10.1007/s00062-015-0430-y.
Rovira A, de Stefano N. MRI monitoring of spinal cord changes in patients with multiple sclerosis. Curr Opin Neurol. 2016;29(4):445–52. doi:10.1097/WCO.0000000000000343.
Gonzalez-Scarano F, Grossman RI, Galetta S, Atlas SW, Silberberg DH. Multiple sclerosis disease activity correlates with gadolinium-enhanced magnetic resonance imaging. Ann Neurol. 1987;21(3):300–6. doi:10.1002/ana.410210312.
Chappell PM, Pelc NJ, Foo TK, Glover GH, Haros SP, Enzmann DR. Comparison of lesion enhancement on spin-echo and gradient-echo images. AJNR Am J Neuroradiol. 1994;15(1):37–44.
Crombe A, Saranathan M, Ruet A, Durieux M, de Roquefeuil E, Ouallet JC, et al. MS lesions are better detected with 3D T1 gradient-echo than with 2D T1 spin-echo gadolinium-enhanced imaging at 3T. AJNR Am J Neuroradiol. 2015;36(3):501–7. doi:10.3174/ajnr.A4152.
Alcaide-Leon P, Pauranik A, Alshafai L, Rawal S, Oh J, Montanera W, et al. Comparison of sagittal FSE T2, STIR, and T1-weighted phase-sensitive inversion recovery in the detection of spinal cord lesions in MS at 3T. AJNR Am J Neuroradiol. 2016;37(5):970–5. doi:10.3174/ajnr.A4656.
• Okuda DT, Siva A, Kantarci O, Inglese M, Katz I, Tutuncu M, et al. Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS One. 2014;9(3):e90509. doi:10.1371/journal.pone.0090509. This retrospective study, which includes the largest number of radiologically isolated syndrome subjects reported to date, identified important factors that increase the risk of developing clinically isolated syndrome or multiple sclerosis.
Okuda DT, Mowry EM, Cree BA, Crabtree EC, Goodin DS, Waubant E, et al. Asymptomatic spinal cord lesions predict disease progression in radiologically isolated syndrome. Neurology. 2011;76(8):686–92. doi:10.1212/WNL.0b013e31820d8b1d.
Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. The Lancet Neurology. 2012;11(2):157–69. doi:10.1016/s1474-4422(11)70274-5.
Brex PA, Ciccarelli O, O’Riordan JI, Sailer M, Thompson AJ, Miller DH. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N Engl J Med. 2002;346(3):158–64. doi:10.1056/NEJMoa011341.
Kuhle J, Disanto G, Dobson R, Adiutori R, Bianchi L, Topping J, et al. Conversion from clinically isolated syndrome to multiple sclerosis: a large multicentre study. Mult Scler. 2015;21(8):1013–24. doi:10.1177/1352458514568827.
Fisniku LK, Brex PA, Altmann DR, Miszkiel KA, Benton CE, Lanyon R, et al. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain. 2008;131(Pt 3):808–17. doi:10.1093/brain/awm329.
Tintore M, Rovira A, Rio J, Nos C, Grive E, Tellez N, et al. Baseline MRI predicts future attacks and disability in clinically isolated syndromes. Neurology. 2006;67(6):968–72. doi:10.1212/01.wnl.0000237354.10144.ec.
Swanton JK, Fernando KT, Dalton CM, Miszkiel KA, Altmann DR, Plant GT, et al. Early MRI in optic neuritis: the risk for disability. Neurology. 2009;72(6):542–50. doi:10.1212/01.wnl.0000341935.41852.82.
Sombekke MH, Wattjes MP, Balk LJ, Nielsen JM, Vrenken H, Uitdehaag BM, et al. Spinal cord lesions in patients with clinically isolated syndrome: a powerful tool in diagnosis and prognosis. Neurology. 2013;80(1):69–75. doi:10.1212/WNL.0b013e31827b1a67.
Tintore M, Rovira A, Arrambide G, Mitjana R, Rio J, Auger C, et al. Brainstem lesions in clinically isolated syndromes. Neurology. 2010;75(21):1933–8. doi:10.1212/WNL.0b013e3181feb26f.
Optic Neuritis Study G. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol. 2008;65(6):727–32. doi:10.1001/archneur.65.6.727.
Swanton JK, Fernando KT, Dalton CM, Miszkiel KA, Altmann DR, Plant GT, et al. Early MRI in optic neuritis: the risk for clinically definite multiple sclerosis. Mult Scler. 2010;16(2):156–65. doi:10.1177/1352458509353650.
•• Tintore M, Rovira A, Rio J, Otero-Romero S, Arrambide G, Tur C, et al. Defining high, medium and low impact prognostic factors for developing multiple sclerosis. Brain. 2015;138(Pt 7):1863–74. doi:10.1093/brain/awv105. This prospective study of over 1000 patients with clinically isolated syndrome assessed a wide range of clinical, radiologic, and biologic prognostic factors and identified low, medium, and high prognostic factors that increase the risk of developing multiple sclerosis and disability accumulation.
Romeo M, Martinelli-Boneschi F, Rodegher M, Esposito F, Martinelli V, Comi G, et al. Clinical and MRI predictors of response to interferon-beta and glatiramer acetate in relapsing-remitting multiple sclerosis patients. Eur J Neurol. 2013;20(7):1060–7. doi:10.1111/ene.12119.
Popescu V, Agosta F, Hulst HE, Sluimer IC, Knol DL, Sormani MP, et al. Brain atrophy and lesion load predict long term disability in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2013;84(10):1082–91. doi:10.1136/jnnp-2012-304094.
Rudick RA, Lee JC, Simon J, Ransohoff RM, Fisher E. Defining interferon beta response status in multiple sclerosis patients. Ann Neurol. 2004;56(4):548–55. doi:10.1002/ana.20224.
•• Sormani MP, Bruzzi P. MRI lesions as a surrogate for relapses in multiple sclerosis: a meta-analysis of randomised trials. The Lancet Neurology. 2013;12(7):669–76. doi:10.1016/s1474-4422(13)70103-0. This meta-analysis of 31 randomized controlled trials in multiple sclerosis confirmed that a disease-modifying therapy’s treatment effect on clinical relapses can be predicted by its effect on MRI lesion activity, supporting the use of MRI measures as primary endpoints in future clinical trials.
Healy BC, Glanz BI, Stankiewicz J, Buckle G, Weiner H, Chitnis T. A method for evaluating treatment switching criteria in multiple sclerosis. Mult Scler. 2010;16(12):1483–9. doi:10.1177/1352458510379245.
Sormani MP, Arnold DL, De Stefano N. Treatment effect on brain atrophy correlates with treatment effect on disability in multiple sclerosis. Ann Neurol. 2014;75(1):43–9. doi:10.1002/ana.24018.
Pelletier D, Garrison K, Henry R. Measurement of whole-brain atrophy in multiple sclerosis. J Neuroimaging. 2004;14(3 Suppl):11S–9S. doi:10.1177/1051228404266264.
Vargas MI, Delavelle J, Kohler R, Becker CD, Lovblad K. Brain and spine MRI artifacts at 3Tesla. J Neuroradiol. 2009;36(2):74–81. doi:10.1016/j.neurad.2008.08.001.
McGowan JC. Technical issues for MRI examination of the spinal cord. J Neurol Sci. 2000;172(Suppl 1):S27–31.
Thorpe JW, Kidd D, Moseley IF, Kenndall BE, Thompson AJ, MacManus DG, et al. Serial gadolinium-enhanced MRI of the brain and spinal cord in early relapsing-remitting multiple sclerosis. Neurology. 1996;46(2):373–8.
Nijeholt GJ, van Walderveen MA, Castelijns JA, van Waesberghe JH, Polman C, Scheltens P, et al. Brain and spinal cord abnormalities in multiple sclerosis. Correlation between MRI parameters, clinical subtypes and symptoms. Brain. 1998;121(Pt 4):687–97.
Lycklama G, Thompson A, Filippi M, Miller D, Polman C, Fazekas F, et al. Spinal-cord MRI in multiple sclerosis. The Lancet Neurology. 2003;2(9):555–62. doi:10.1016/s1474-4422(03)00504-0.
Rovaris M, Mastronardo G, Prandini F, Bastianello S, Comi G, Filippi M. Short-term evolution of new multiple sclerosis lesions enhancing on standard and triple dose gadolinium-enhanced brain MRI scans. J Neurol Sci. 1999;164(2):148–52.
Cotton F, Weiner HL, Jolesz FA, Guttmann CRG. MRI contrast uptake in new lesions in relapsing-remitting MS followed at weekly intervals. Neurology. 2003;60(4):640–6. doi:10.1212/01.wnl.0000046587.83503.1e.
Thomsen HS, Morcos SK, Almen T, Bellin MF, Bertolotto M, Bongartz G, et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol. 2013;23(2):307–18. doi:10.1007/s00330-012-2597-9.
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834–41. doi:10.1148/radiol.13131669.
Murata N, Murata K, Gonzalez-Cuyar LF, Maravilla KR. Gadolinium tissue deposition in brain and bone. Magn Reson Imaging. 2016;34(10):1359–65. doi:10.1016/j.mri.2016.08.025.
Stojanov DA, Aracki-Trenkic A, Vojinovic S, Benedeto-Stojanov D, Ljubisavljevic S. Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol. 2016;26(3):807–15. doi:10.1007/s00330-015-3879-9.
Kanda T, Osawa M, Oba H, Toyoda K, Kotoku J, Haruyama T, et al. High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology. 2015;275(3):803–9. doi:10.1148/radiol.14140364.
Radbruch A, Weberling LD, Kieslich PJ, Eidel O, Burth S, Kickingereder P, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology. 2015;275(3):783–91. doi:10.1148/radiol.2015150337.
Robert P, Lehericy S, Grand S, Violas X, Fretellier N, Idee JM, et al. T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Investig Radiol. 2015;50(8):473–80. doi:10.1097/RLI.0000000000000181.
Rio J, Castillo J, Rovira A, Tintore M, Sastre-Garriga J, Horga A, et al. Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler. 2009;15(7):848–53. doi:10.1177/1352458509104591.
Sormani MP, Rio J, Tintore M, Signori A, Li D, Cornelisse P, et al. Scoring treatment response in patients with relapsing multiple sclerosis. Mult Scler. 2013;19(5):605–12. doi:10.1177/1352458512460605.
Romeo M, Martinelli V, Rodegher M, Perego E, Maida S, Sormani MP, et al. Validation of 1-year predictive score of long-term response to interferon-beta in everyday clinical practice multiple sclerosis patients. Eur J Neurol. 2015;22(6):973–80. doi:10.1111/ene.12695.
Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet (London, England). 1998;352(9139):1498–504.
Sormani MP, De Stefano N. Defining and scoring response to IFN-beta in multiple sclerosis. Nat Rev Neurol. 2013;9(9):504–12. doi:10.1038/nrneurol.2013.146.
•• Freedman MS, Selchen D, Arnold DL, Prat A, Banwell B, Yeung M, et al. Treatment optimization in MS: Canadian MS Working Group updated recommendations. The Canadian Journal of Neurological Sciences. 2014;40(03):307–23. doi:10.1017/s0317167100014244. This review provides a practical approach to assessing treatment response in patients with multiple sclerosis and a strategy for switching amongst available disease-modifying treatments.
Yong VW. Differential mechanisms of action of interferon-β and glatiramer acetate in MS. Neurology. 2002;59(6):802–8. doi:10.1212/wnl.59.6.802.
Stone LA, Frank JA, Albert PS, Bash CN, Calabresi PA, Maloni H, et al. Characterization of MRI response to treatment with interferon beta-1b: contrast-enhancing MRI lesion frequency as a primary outcome measure. Neurology. 1997;49(3):862–9.
Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging–measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer acetate study group Ann Neurol 2001;49(3):290–297.
Vagberg M, Axelsson M, Birgander R, Burman J, Cananau C, Forslin Y, et al. Guidelines for the use of magnetic resonance imaging in diagnosing and monitoring the treatment of multiple sclerosis: recommendations of the Swedish Multiple Sclerosis Association and the Swedish Neuroradiological Society. Acta Neurol Scand. 2017;135(1):17–24. doi:10.1111/ane.12667.
Tremlett H, Zhao Y, Joseph J, Devonshire V, Neurologists UC. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry. 2008;79(12):1368–74. doi:10.1136/jnnp.2008.145805.
Tan CS, Koralnik IJ. Progressive multifocal leukoencephalopathy and other disorders caused by JC virus: clinical features and pathogenesis. Lancet Neurol. 2010;9(4):425–37. doi:10.1016/S1474-4422(10)70040-5.
Kappos L, Bates D, Edan G, Eraksoy M, Garcia-Merino A, Grigoriadis N, et al. Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. The Lancet Neurology. 2011;10(8):745–58. doi:10.1016/s1474-4422(11)70149-1.
Faulkner M. Risk of progressive multifocal leukoencephalopathy in patients with multiple sclerosis. Expert Opin Drug Saf. 2015;14(11):1737–48. doi:10.1517/14740338.2015.1093620.
Gyang TV, Hamel J, Goodman AD, Gross RA, Samkoff L. Fingolimod-associated PML in a patient with prior immunosuppression. Neurology. 2016;86(19):1843–5. doi:10.1212/WNL.0000000000002654.
Nieuwkamp DJ, Murk JL, van Oosten BW, Cremers CH, Killestein J, Viveen MC, et al. PML in a patient without severe lymphocytopenia receiving dimethyl fumarate. N Engl J Med. 2015;372(15):1474–6. doi:10.1056/NEJMc1413724.
Dong-Si T, Richman S, Wattjes MP, Wenten M, Gheuens S, Philip J, et al. Outcome and survival of asymptomatic PML in natalizumab-treated MS patients. Ann Clin Transl Neurol. 2014;1(10):755–64. doi:10.1002/acn3.114.
McGuigan C, Craner M, Guadagno J, Kapoor R, Mazibrada G, Molyneux P, et al. Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry. 2016;87(2):117–25. doi:10.1136/jnnp-2015-311100.
Wijburg MT, Witte BI, Vennegoor A, Roosendaal SD, Sanchez E, Liu Y, et al. MRI criteria differentiating asymptomatic PML from new MS lesions during natalizumab pharmacovigilance. J Neurol Neurosurg Psychiatry. 2016;87(10):1138–45. doi:10.1136/jnnp-2016-313772.
Taieb G, Renard D, Thouvenot E, Servillo G, Castelnovo G. Transient punctuate enhancing lesions preceding natalizumab-associated progressive multifocal leukoencephalopathy. J Neurol Sci. 2014;346(1–2):364–5. doi:10.1016/j.jns.2014.09.007.
Giacomini PS, Rozenberg A, Metz I, Araujo D, Arbour N, Bar-Or A. Maraviroc and JC virus-associated immune reconstitution inflammatory syndrome. N Engl J Med. 2014;370(5):486–8. doi:10.1056/NEJMc1304828.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
David Kim declares that he has no conflict of interest.
Suradech Suthiphosuwan has received grants from Sanofi-Genzyme.
Aditya Bharatha has received honorarium for education lectures from Novartis, Biogen, and EMD Serono.
Jiwon Oh has received a grant from the MS Society of Canada, the National MS Society, grants and consultation fees from Biogen-Idec and Sanofi-Genzyme, and consultation fees from EMD Serono, Novartis, Roche, and Teva.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Multiple Sclerosis and Related Disorders
Rights and permissions
About this article
Cite this article
Suthiphosuwan, S., Kim, D., Bharatha, A. et al. Imaging Markers for Monitoring Disease Activity in Multiple Sclerosis. Curr Treat Options Neurol 19, 18 (2017). https://doi.org/10.1007/s11940-017-0453-6
Published:
DOI: https://doi.org/10.1007/s11940-017-0453-6