Opinion statement
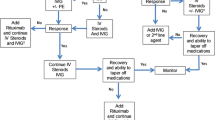
The timely implementation of immunotherapy is key to successful treatment of autoimmune encephalopathies or dementias (from here on will be referred to as autoimmune encephalopathies). There are different levels of diagnostic certainty which should guide the immunological treatment of autoimmune encephalopathies. There is a high level of diagnostic certainty for patients who have classic limbic encephalitis and have a neural antibody detected in serum or CSF (such as potassium channel complex antibody). For these patients, initiating high-dose corticosteroids or IVIg is indicated, with plasma exchange, rituximab or cyclophosphamide used as second-line therapy if first-line therapy proves only partially beneficial. There is a lower level of diagnostic certainty in patients with non-limbic atypical phenotypes (though rapidly progressive) when no neural antibody is detected in serum and CSF. A trial of corticosteroids or IVIg (or both sequentially) may be undertaken in these patients, but if no objective improvements occur, further immunotherapy is unlikely to be beneficial. Antiepileptic treatment also plays a critical role in those who have seizures as well as cognitive symptoms. Evaluation for and treatment of any underlying cancer is another component for those patients with a paraneoplastic cause of encephalitis. An individualized maintenance regimen needs to be designed for patients who do improve with immunotherapy. Individual factors that need to be considered when formulating a program of maintenance treatment include disease severity, antibody specificity and proclivity for disease relapse. Azathioprine and mycophenolate mofetil are frequently used for the purpose of remission maintenance, and should permit gradual withdrawal of steroids, IVIg or more toxic immunosuppressants. The duration of maintenance therapy is uncertain, but this author typically recommends 3–5 years of relapse-free maintenance treatment before discontinuing immunotherapy altogether.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Flanagan EP, McKeon A, Lennon VA, et al. Autoimmune dementia: clinical course and predictors of immunotherapy response. Mayo Clin Proc. 2010;85:881–97. This article describes the spectrum of autoimmune encephalopathies seen at Mayo Clinic. As well as clinical descriptions, serological findings, and predictors of immunotherapy response are discussed.
McKeon A, Lennon VA, Pittock SJ. Immunotherapy-responsive dementias and encephalopathies. Continuum (Minneap Minn). 2010;16:80–101. This describes in detail the evaluation of patients with suspected autoimmune encephalopathy.
Geschwind MD, Tan KM, Lennon VA, et al. Voltage-gated potassium channel autoimmunity mimicking creutzfeldt-jakob disease. Arch Neurol. 2008;65:1341–6.
Castillo P, Woodruff B, Caselli R, et al. Steroid-responsive encephalopathy associated with autoimmune thyroiditis. Arch Neurol. 2006;63:197–202.
McKeon A, Pittock SJ, Lennon VA. CSF complements serum for evaluating paraneoplastic antibodies and NMO-IgG. Neurology. 2011;76:1108–10.
Gultekin SH, Rosenfeld MR, Voltz R, et al. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123(Pt 7):1481–94.
Vernino S, O'Neill BP, Marks RS, et al. Immunomodulatory treatment trial for paraneoplastic neurological disorders. Neuro Oncol. 2004;6:55–62.
Vincent A, Buckley C, Schott JM, et al. Potassium channel antibody-associated encephalopathy: a potentially immunotherapy-responsive form of limbic encephalitis. Brain. 2004;127:701–12.
Lai M, Huijbers MG, Lancaster E, et al. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol. 2010;9:776–85.
McKeon A, Lennon VA, Pittock SJ. Immunotherapy-responsive dementias and encephalopathies. Continuum Lifelong Learn Neurol. 2010;16:80–101.
Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–65. This is an important detailed study of the benefits of immunotherapy for NMDA R encephalitis. The authors describe 1st ine and 2nd line therapy.
Quek AM, Britton JW, McKeon A et al. Autoimmune Epilepsy: Clinical Characteristics and Response to Immunotherapy. Arch Neurol. 2012.
Oakley RH, Cidlowski JA. Cellular processing of the glucocorticoid receptor gene and protein: new mechanisms for generating tissue-specific actions of glucocorticoids. J Biol Chem. 2011;286:3177–84.
Byyny RL. Withdrawal from glucocorticoid therapy. N Engl J Med. 1976;295:30–2.
Green H, Paul M, Vidal L, Leibovici L. Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82:1052–9.
Sangiolo D, Storer B, Nash R, et al. Toxicity and efficacy of daily dapsone as Pneumocystis jiroveci prophylaxis after hematopoietic stem cell transplantation: a case-control study. Biol Blood Marrow Transplant. 2005;11:521–9.
Tomonari A, Takahashi S, Ooi J, et al. No occurrence of Pneumocystis jiroveci (carinii) pneumonia in 120 adults undergoing myeloablative unrelated cord blood transplantation. Transpl Infect Dis. 2008;10:303–7.
Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res. 2010;62:1515–26.
Richards RN. Short-term corticosteroids and avascular necrosis: medical and legal realities. Cutis. 2007;80:343–8.
Dubovsky AN, Arvikar S, Stern TA, Axelrod L. The neuropsychiatric complications of glucocorticoid use: steroid psychosis revisited. Psychosomatics. 2012;53:103–15.
Abu-Shakra M. Safety of vaccination of patients with systemic lupus erythematosus. Lupus. 2009;18:1205–8.
Gelfand EW. Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med. 2012;367:2015–25.
Burks AW, Sampson HA, Buckley RH. Anaphylactic reactions after gamma globulin administration in patients with hypogammaglobulinemia. Detection of IgE antibodies to IgA. N Engl J Med. 1986;314:560–4.
Ford LT, Berg JD. Thiopurine S-methyltransferase (TPMT) assessment prior to starting thiopurine drug treatment; a pharmacogenomic test whose time has come. J Clin Pathol. 2010;63:288–95.
Costanzi C, Matiello M, Lucchinetti CF, et al. Azathioprine: tolerability, efficacy, and predictors of benefit in neuromyelitis optica. Neurology. 2011;77:659–66.
Sanderson J, Ansari A, Marinaki T, Duley J. Thiopurine methyltransferase: should it be measured before commencing thiopurine drug therapy? Ann Clin Biochem. 2004;41:294–302.
Kandiel A, Fraser AG, Korelitz BI, et al. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121–5.
Witte AS, Cornblath DR, Schatz NJ, Lisak RP. Monitoring azathioprine therapy in myasthenia gravis. Neurology. 1986;36:1533–4.
Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000;47:85–118.
Jacob A, Matiello M, Weinshenker BG, et al. Treatment of neuromyelitis optica with mycophenolate mofetil: retrospective analysis of 24 patients. Arch Neurol. 2009;66:1128–33.
Jacob A, Weinshenker BG, Violich I, et al. Treatment of neuromyelitis optica with rituximab: retrospective analysis of 25 patients. Arch Neurol. 2008;65:1443–8.
Molloy ES, Calabrese LH. Progressive multifocal leukoencephalopathy associated with immunosuppressive therapy in rheumatic diseases: evolving role of biologic therapies. Arthritis Rheum. 2012;64:3043–51.
McKeon A, Pittock, SJ. Individualized Rituximab Treatment for Neuromyelitis Optica Spectrum Disorders JAMA Neurol. 2013
Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42.
Gourley MF, Austin 3rd HA, Scott D, et al. Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis. A randomized, controlled trial. Ann Intern Med. 1996;125:549–57.
Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349:36–44.
Allopurinol and cytotoxic drugs. Interaction in relation to bone marrow depression. Boston Collaborative Drug Surveillance Program. JAMA. 1974;227:1036–1040
Park MC, Park YB, Jung SY, et al. Risk of ovarian failure and pregnancy outcome in patients with lupus nephritis treated with intravenous cyclophosphamide pulse therapy. Lupus. 2004;13:569–74.
Lucchinetti CF, Kimmel DW, Lennon VA. Paraneoplastic and oncologic profiles of patients seropositive for type 1 antineuronal nuclear autoantibodies. Neurology. 1998;50:652–7.
Pittock SJ, Lucchinetti CF, Lennon VA. Anti-neuronal nuclear autoantibody type 2: paraneoplastic accompaniments. Ann Neurol. 2003;53:580–7.
Luque FA, Furneaux HM, Ferziger R, et al. Anti-Ri: an antibody associated with paraneoplastic opsoclonus and breast cancer. Ann Neurol. 1991;29:241–51.
Chan KH, Vernino S, Lennon VA. ANNA-3 anti-neuronal nuclear antibody: marker of lung cancer-related autoimmunity. Ann Neurol. 2001;50:301–11.
Vernino S, Lennon VA. New Purkinje cell antibody (PCA-2): marker of lung cancer-related neurological autoimmunity. Ann Neurol. 2000;47:297–305.
Pittock SJ, Lucchinetti CF, Parisi JE, et al. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann Neurol. 2005;58:96–107.
Yu Z, Kryzer TJ, Griesmann GE, et al. CRMP-5 neuronal autoantibody: marker of lung cancer and thymoma-related autoimmunity. Ann Neurol. 2001;49:146–54.
Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain. 2004;127:1831–44.
Dalmau J, Gultekin SH, Voltz R, et al. Ma1, a novel neuron- and testis-specific protein, is recognized by the serum of patients with paraneoplastic neurological disorders. Brain. 1999;122(Pt 1):27–39.
Graus F, Vincent A, Pozo-Rosich P, et al. Anti-glial nuclear antibody: marker of lung cancer-related paraneoplastic neurological syndromes. J Neuroimmunol. 2005;165:166–71.
Klein CJ, Lennon VA, Aston PA, et al. Insights from LGI1 and CASPR2 potassium channel complex autoantibody subtyping. JAMA Neurol. 2013;70:229–34.
Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36.
Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9:67–76.
Boronat A, Sabater L, Saiz A, et al. GABA(B) receptor antibodies in limbic encephalitis and anti-GAD-associated neurologic disorders. Neurology. 2011;76:795–800.
Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65:424–34.
McKeon A, Lennon VA, Lachance DH, et al. Ganglionic acetylcholine receptor autoantibody: oncological, neurological, and serological accompaniments. Arch Neurol. 2009;66:735–41.
Lancaster E, Martinez-Hernandez E, Titulaer MJ, et al. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology. 2011;77:1698–701.
Compliance with Ethics Guidelines
Conflict of Interest
Andrew McKeon has received grant support from the Guthy Jackson Charitable Foundation.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McKeon, A. Immunotherapeutics for Autoimmune Encephalopathies and Dementias. Curr Treat Options Neurol 15, 723–737 (2013). https://doi.org/10.1007/s11940-013-0251-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11940-013-0251-8