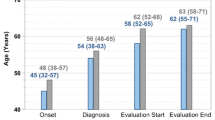
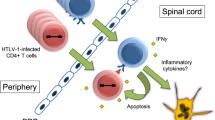
Opinion statement
Human T-lymphotrophic virus 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is a disabling myelopathy, but clinical trials of specific drugs to treat it are lacking. There are many reasons for the absence of specific therapeutic studies, including difficulty in enrolling patients, inadequate measurement tools to evaluate neurologic improvement, and even lack of interest. Oral or intravenous corticosteroids are now the mainstay of HAM/TSP treatment, especially in the initial phase of the disease, when inflammation is more prominent than demyelination. Motor disability, pain, and urinary dysfunction may be ameliorated, but improvement is not sustained in many patients. Valproic acid has emerged as a potential treatment for HAM/TSP; recent evidence shows that this drug can activate viral gene expression and expose virus-infected cells to the immune system, leading to a reduction of the proviral load. Alternative drugs such as methotrexate, pentoxifylline, azathioprine, danazol, and interferon-α may be used if steroids fail or cannot be tolerated, but they have not been assessed in randomized clinical trials.
Similar content being viewed by others
References and Recommended Reading
Araújo A, Silva MTT: The HTLV-1 neurological complex. Lancet Neurol 2006, 5:1068–1076.
Silva MTT, Harab RC, Leite AC, et al.: Human T lymphotropic virus type 1 (HTLV-1) proviral load in asymptomatic carriers, HTLV-1-associated myelopathy/tropical spastic paraparesis, and other neurological abnormalities associated with HTLV-1 infection. Clin Infect Dis 2007, 44:689–692.
Cartier L, Cea JG, Vergara C, et al.: Clinical and neuropathological study of six patients with spastic paraparesis associated with HTLV-I: an axomyelinic degeneration of the central nervous system. J Neuropathol Exp Neurol 1997, 56:403–413.
Iwasaki Y, Ohara Y, Kobayashi I, Akizuki S: Infiltration of helper/inducer T lymphocytes heralds central nervous system damage in human T-cell leukemia virus infection. Am J Pathol 1992, 140:1003–1008.
Nakagawa M, Nakahara K, Maruyama Y, et al.: Therapeutic trials in 200 patients with HTLV-I-associated myelopathy/tropical spastic paraparesis. J Neurovirol 1996, 2:345–355.
Araújo AQ, Afonso CR, Leite AC, Dultra SV: Intravenous methylprednisolone in HTLV-I associated myelopathy/tropical spastic paraparesis (HAM/TSP). Arq Neuropsiquiatr 1993, 51:325–328.
Yamasaki K, Kira J, Koyanagi Y, et al.: Long-term, high dose interferon-alpha treatment in HTLV-I-associated myelopathy/tropical spastic paraparesis: a combined clinical, virological and immunological study. J Neurol Sci 1997, 147:135–144.
Izumo S, Goto I, Itoyama Y, et al.: Interferon-alpha is effective in HTLV-I-associated myelopathy: a multicenter, randomized, double-blind, controlled trial. Neurology 1996, 46:1016–1021.
Kuroda Y, Kurohara K, Fujiyama F, et al.: Systemic interferon-alpha in the treatment of HTLV-I-associated myelopathy. Acta Neurol Scand 1992, 86:82–86.
Isono T, Ogawa K, Seto A: Antiviral effect of zidovudine in the experimental model of adult T-cell leukaemia in rabbits. LeukaemiaResearch 1990, 14:841–847.
Gout O, Gessain A, Iba-Zizen M, et al.: The effect of zidovudine on chronic myelopathy associated with HTLV-1. J Neurol 1991, 238:108–109.
Sheremata WA, Benedict BS, Squilacote DC, et al.: High-dose zidovudine induction in HTLV-I associated myelopathy: safety and possible efficacy. Neurology 1993, 43:2125–2129.
Taylor GP, Hall SE, Navarrete S, et al.: Effect of lamivudine on human T-cell leukemia virus type 1 (HTLV-1) DNA copy number, T-cell phenotype, and anti-Tax cytotoxic T-cell frequency in patients with HTLV-1-associated myelopathy. J Virol 1999, 73:10289–10295.
Taylor G, Goon P, Furukawa Y, et al.: Zidovudine plus lamivudine in human T-lymphotropic virus type-I-associated myelopathy: a randomised trial. Retrovirology 2006, 3:63.
Nakamura T, Shirabe S, Ichinose K, et al.: Pentoxifylline treatment in HTLV-I-associated myelopathy [letter]. Intern Med 1995, 34:460.
Shirabe S, Nakamura T, Tsujino A, et al.: Successful application of pentoxifylline in the treatment of HTLV-I associated myelopathy. J Neurol Sci 1997, 151:97–101.
Harrington WJ, Sheremata WA, Snodgrass SR, et al.: Tropical spastic paraparesis/HTLV-1-associated myelopathy (TSP/HAM): treatment with an anabolic steroid danazol. AIDS Res Hum Retroviruses 1991, 7:1031–1034.
Melo A, Moura L, Meireles A, Costa G: Danazol. A new perspective in the treatment of HTLV-1 associated myelopathy (preliminary report). Arq Neuropsiquiatr 1992, 50:402–403.
Cronstein BN: Going with the flow: methotrexate, adenosine, and blood flow. Ann Rheum Dis 2006, 65:421–422.
Li HC, Yashiki S, Sonoda J, et al.: Green tea polyphenols induce apoptosis in vitro in peripheral blood T lymphocytes of adult T-cell leukemia patients. Jpn J Cancer Res 2000, 91:34–40.
Sonoda J, Koriyama C, Yamamoto S, et al.: HTLV-1 provirus load in peripheral blood lymphocytes of HTLV-1 carriers is diminished by green tea drinking. Cancer Sci 2004, 95:596–601.
Kato I, Yokokura T, Mutai M: Augmentation of mouse natural killer cell activity by Lactobacillus casei and its surface antigens. Microbiol Immunol 1984, 27:209–217.
Matsuzaki T, Saito M, Usuku K, et al.: Prospective uncontrolled trial of fermented milk drink containing viable Lactobacillus casei strain Shirota in the treatment of HTLV-1 associated myelopathy/tropical spastic paraparesis. J Neurol Sci 2005, 237:75–81.
Oh U, Yamano Y, Mora CA, et al.: Interferon-β1a therapy in human T-lymphotropic virus type I-associated neurologic disease. Ann Neurol 2005, 57:526–534.
Lezin A, Gillet N, Olindo S, et al.: Histone deacetylase mediated transcriptional activation reduces proviral loads in HTLV-1-associated myelopathy/tropical spastic paraparesis patients. Blood 2007, 110:3722–3728.
Araújo AP, Fontenelle LM, Padua PA, et al.: Juvenile human T lymphotropic virus type 1-associated myelopathy. Clin Infect Dis 2002, 35:201–204.
Bittencourt AL, Primo J, Oliveira MF: Manifestations of the human T-cell lymphotropic virus type I infection in childhood and adolescence. J Pediatr (Rio J) 2006, 82:411–420.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Araújo, A., Lima, M.A. & Silva, M.T.T. Human T-lymphotropic virus 1 neurologic disease. Curr Treat Options Neurol 10, 193–200 (2008). https://doi.org/10.1007/s11940-008-0021-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11940-008-0021-1