Abstract
Purpose of review
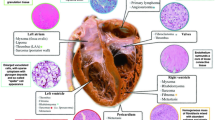
Modern cancer therapies have resulted in prolonged survival and decreased mortality for many types of cancer. Consequently, cancer patients with concomitant cardiovascular disease (CVD) present prior to diagnosis of cancer, resulting from various forms of cancer therapy, or developing after cancer remission are at higher risk for development of cardiovascular-associated morbidity and mortality. Management of CVD, particularly structural heart disease, in this patient population is challenging and associated with elevated surgical risk. Minimally invasive transcatheter procedures have emerged as lower risk treatment modalities that have been shown to be safe and efficacious. This review will evaluate the current literature on percutaneous management of aortic stenosis (AS), mitral regurgitation (MR), tricuspid regurgitation (TR), and intracardiac thrombus (ICTE) or masses in cancer patients.
Recent findings
Multiple cohort- and registry-based studies have demonstrated safe and efficacious implementation of transcatheter interventions for the management of severe AS and MR in cancer patients. The development of transcatheter interventions for severe TR is promising and may also be considered for this high-risk patient population. Interestingly, management of ICTE and cardiac masses has also been achieved with a novel transcatheter approach.
Summary
While a multidisciplinary heart team approach is necessary to properly manage cancer patients with structural heart disease and ICTE, more robust registry-based and prospective clinical trials are required to meet the needs of a rapidly growing cardio-oncology patient population.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Xu J, Murphy SL, et al. Mortality in the United States, 2018. NCHS Data Brief. 2020;355.
• Curigliano G, Cardinale D, Dent S, et al. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin. 2016; 66: 309–25. Extensive review on cardiotoxicity of various forms of cancer therapy.
Ball S, Ghosh R, Wongsaengsak S, et al. Cardiovascular toxicities of immune checkpoint inhibitors. JACC; 74: 1714–27.
•• Pushparaji B, Marmagkiolis K, Miller C, et al. State-of-the-art review: interventional onco-cardiology. Curr Treat Options Cardio Med. 2020;22:11 Review of interventional cardiology in cancer patients that includes a wide spectrum of procedures and provides important retrospective literature.
Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular complications of cancer therapy. Best practices in diagnosis, prevention, and management: part 1. JACC. 2017;70:2536–51.
Al-Kindi S, Oliveira G. Prevalence of preexisting cardiovascular disease in patients with different types of cancer: the unmet need for onco-cardiology. Mayo Clinic Proc. 2016;90:81–3.
• Desai MY, Windecker S, et al. Prevention, diagnosis, and management of radiation-associated cardiac disease. J Am Coll Cardiol. 2019; 74: 905–27. Provided pathophysiologic etiology of radiation-induced valvular disease and extent of cardiac injury.
Gujral DM, Lloyd G, Bhattacharyya S. Radiation-induced valvular heart disease. Heart. 2016;102:269–76.
•• Crestanello JA, McGregor CGA, et al. Mitral and tricuspid valve repair in patients with previous mediastinal radiation therapy. Ann Thorac Surg. 2004; 76: 826–31. Key initial literature documenting the poor surgical outcomes in patients with radiation-induced valvular disease.
Nadlonek NA, Weyant MJ, Yu JA, Cleveland JC Jr, Reece TB, Meng X, et al. Radiation induces osteogenesis in the human aortic valve interstitial cells. J Thorac Cardiovasc Surg. 2012;144:1466–70.
Mukai M, Oka T. Mechanism and management of cancer-associated thrombosis. J Cardiol. 2018;72:89–93.
Herrmann J, Yang EH, Iliescu CA, Cilingiroglu M, Charitakis K, Hakeem A, et al. Vascular toxicities of cancer therapies, the old and the new – an evolving avenue. Circulation. 2016;133:1272–89.
Christiansen S, Schmid C, Löher A, Scheld HH. Impact of malignant hematologic disorders on cardiac surgery. Cardiovasc Surg. 2000;8:149–52.
Chan J, Rosenfeldt F, Chaudhuri K, Marasco S. Cardiac surgery in patients with history of malignancy: increased complication rate but similar mortality. Heart, Lung, and Circulation. 2012;21:255–9.
• Gill C, Lee M, et al. Transcatheter and surgical aortic valve replacement impact on outcomes and cancer treatment schedule. Int J Cardiol. 2020; 20: 33645–8. Recent data comparing surgical and aortic valve replacement as it pertains to cancer patients and initiation of their cancer therapies.
Samuels LE, Kaufman MS, Morris RJ, Styler M, Brockman SK. Open heart surgery in patients with chronic lymphocytic leukemia. Leuk Res. 1999;23:71–5.
Carrascal Y, Gualis J, Arévalo A, Fulquet E, Flórez S, Rey J, et al. Cardiac surgery with extracorporeal circulation in cancer patients: influence on surgical morbidity and mortality and on survival. Rev Esp Cardiol. 2008;61:369–75.
•• Yusuf SW, Sarafarz A, et al. Management and outcomes of severe aortic stenosis in cancer patients. Am Heart J. 2011; 161: 1125–32. First retrospective study evaluating severe AS outcomes in the cancer population.
Berkovitch A, Guetta V, Barbash IM, Fink N, Regev E, Maor E, et al. Favorable short-term and long-term outcomes among patients with prior history of malignancy undergoing transcatheter aortic valve implantation. J Invasive Cardiol. 2018;30:105–9.
• Leon MB, Smith CR, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010; 363: 1597–1607. Seminal randomized control trial evaluating transcatheter aortic valve replacement in patients with prohibitive surgical risk.
Leon MB, Smith CR, et al. Transcatheter aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–98.
Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609–20.
Mack MJ, Lean MB, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1665–705.
•• Nishimura RA, Otto CM, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease. Circulation. 2017; 135: 159–195. Most recent recommendations for the management of valvular heart disease.
Faggiano P, Frattini S, et al. Prevalence of comorbidities and associated cardiac diseases in patients with valve aortic stenosis. Potential implications for the decision-making process. Int J Cardiol. 2012;150:94–9.
Minamino-Muta E, Kato T, Morimoto T, Taniguchi T, Nakatsuma K, Kimura Y, et al. Malignant disease as a comorbidity in patients with severe aortic stenosis: clinical presentation, outcomes, and management. Eur Heart J Qual Care Clin Outcomes. 2018;4:180–8.
Mangner N, Woitek FJ, Haussig S, Holzhey D, Stachel G, Schlotter F, et al. Impact of active cancer disease on the outcome of patients undergoing transcatheter aortic valve replacement. J Interv Cardiol. 2018;31(2):188–96.
Watanabe Y, Kozuma K, Hioki H, Kawashima H, Nara Y, Kataoka A, et al. Comparison of results of transcatheter aortic valve implantation inpatients with versus without active cancer. Am J Cardiol. 2016;118(4):572–7.
•• Landes U, Iakobishvili Z, et al. Transcatheter aortic valve replacement in oncology patients with severe aortic stenosis. JACC Cardiovasc Interv. 2019;12(1):78–86. Largest registry-based study evaluating transcatheter aortic valve replacement outcomes in cancer patients.
Lind A, Totzeck M, Mahabadi AA, et al. Impact of cancer in patients undergoing transcatheter aortic valve replacement: a single-center study. J Am Coll Cardiol CardioOnc. 2020:735–43.
Online STS adult cardiac surgery risk calculator, 2020, riskcalc.sts.org/stswebriskcalc/calculate. Last accessed December 26th, 2020.
• Balanescu SM, Balanescu DV, et al. The onco-cardiologist dilemma: to implant, to defer, or to avoid transcatheter aortic valve replacement in cancer patients with aortic stenosis? Current Cardiology Reports. 2019; 21: 83. Extensive review of implantation of transcatheter aortic valve replacement in the cancer population.
Donnellan E, Masri A, et al. Long-term outcomes of patients with mediastinal radiation–associated severe aortic stenosis and subsequent surgical aortic valve replacement: a matched cohort study. J Am Heart Assoc. 2017;6:e005396.
Donnellan E, Krishnaswamy A, Hutt-Centeno E, Johnston DR, Aguilera J, Kapadia SR, et al. Outcomes of patients with mediastinal radiation-associated severe aortic stenosis undergoing transcatheter aortic valve replacement. Circulation. 2018;138:1752–4.
• Dijos M, Reynaud A, et al. Efficacy and follow-up of transcatheter aortic valve implantation inpatients with radiation-induced aortic stenosis. Open Heart. 2015; 2: 1. One of only a few prospective trials evaluating the surgical versus transcatheter aortic valve replacement.
Arora S, Lahewala S, et al. Transcatheter aortic valve replacement in aortic regurgitation: the U.S. experience. Catheter Cardiovasc Interv. 2020;1–10. https://doi.org/10.1002/ccd.29379.
• Feldman T, Foster E, et al. Percutaneous repair of surgery for mitral regurgitation. N Engl J Med. 2011; 364: 1395–496. Seminal randomized control trial evaluating the implementation of percutaneous mitral valve repair.
Stone GW, Lindenfield JA, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–18.
Zuern CS, Bauer A, Lubos E, Boekstegers P, Puls M, Bardeleben RS, et al. Influence of non-cardiac comorbidities on outcome after percutaneous mitral valve repair: results from the German transcatheter mitral valve interventions (TRAMI) registry. Clin Res Cardiol. 2015;104:1044–53.
•• Guha A, Dey AK, et al. Contemporary trends and outcomes of percutaneous and surgical mitral valve replacement or repair in patients with cancer. Am J Cardiol. 2020; 125: 1355–60. First large registry-based study evaluating percutaneous mitral valve repair in cancer patients.
Donnellan E, Alashi A, et al. Outcomes of patients with mediastinal radiation-associated mitral valve disease undergoing cardiac surgery. Circulation. 2019;149:1288–90.
Scarfo I, Denti P, et al. MitraClip for radiotherapy-related mitral valve regurgitation. Hell J Cardiol. 2019;60:232–8.
Nishimura RA, Otto CM, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary. Circulation. 2014;129:1440–92.
Prihadi EA, Delgado V, Leon MB, Enriquez-Sarano M, Topilsky Y, Bax JJ. Morphologic types of tricuspid regurgitation: characteristics and prognostic implications. J Am Coll Cardiol Img. 2019;12:491–9.
Tang GH. “Tricuspid clip in tricuspid regurgitation.” JACC. 2020. https://www.acc.org/latest-in-cardiology/articles/2020/02/21/08/26/tricuspid-clip-in-tricuspid-regurgitation. Accessed 3 November 2020.
Asmarats L, Perlman G, et al. Long-term outcomes of the FORMA transcatheter tricuspid valve repair system for the treatment of severe tricuspid regurgitation. J Am Coll Cardiol Intv. 2019;1(2):1438–47.
Mehr M, Taramasso M, et al. 1-year outcomes after edge-to-edge valve repair of symptomatic tricuspid regurgitation. J Am Coll Cardiol Intv. 2019;12:1451–61.
Nickenig G, Weber M, Lurz P, von Bardeleben RS, Sitges M, Sorajja P, et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet. 2019;394:2002–11.
• Donaldson CW, Baker JN, et al. Thrombectomy using suction filtration and veno-venous bypass: single center experience with a novel device. Catheterization and Cardiovascular Interventions. 2015; 86: e81–87. First case series evaluating the use of AngioVac.
Koć M, Kostrubiec M, et al. Outcome of patients with right heart thrombi: the Right Heart Thrombi European Registry. Eur Respir J. 2016; 47: 869–75.
• Moriarty JM, Rueda V, et al. Endovascular removal of thrombus and right heart masses using the AngioVac system: results of 234 patients from the Prospective, Multicenter Registry of AngioVac Procedures in Detail (RAPID). J Vasc Intervl Radiol. 2020. In press. First registry-based study evaluating the use of AngioVac.
Moriarty JM, Al-Hakim R, et al. Removal of Caval and right atrial thrombi and masses using the AngioVac device: initial operative experience. J Vasc Interv Radiol. 2016;27:1584–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Conflict of Interest
Daniel J. Jimenez declares that he has no conflict of interest. Rushi V. Parikh receives grant support from the American Heart Association and Janssen Pharmaceuticals Inc. He is an advisor to Stallion Cardio, DocVocate, and HeartCloud. He declares no relevant conflict of interest. Megan Kamath declares that she has no conflict of interest. Marcella Calfon-Press declares that she has no conflict of interest. John M. Moriarty receives grant funding from Angiodynamics Inc. He is on the advisory board of Angiodynamics Inc. and Boston Scientific Inc. He is also a consultant for BD Bard, PAVMED Inc., Thrombex Inc., and Penumbra Inc. Olcay Aksoy declares that he has no conflict of interest. Nicolas Palaskas declares that he has no conflict of interest. Juan Lopez-Mattei declares that he has no conflict of interest. Cezar A. Iliescu declares that he has no conflict of interest. Eric H. Yang receives research funding from CSL Behring. He declares no relevant conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardio-Oncology
Rights and permissions
About this article
Cite this article
Jimenez, D.J., Parikh, R.V., Kamath, M. et al. Structural Transcatheter Cardiac Interventions in the Cardio-Oncology Population. Curr Treat Options Cardio Med 23, 20 (2021). https://doi.org/10.1007/s11936-021-00898-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s11936-021-00898-2