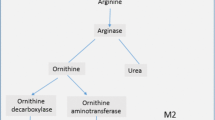
Abstract
Purpose of Review
Macrophages play key roles in tissue homeostasis and immune surveillance, mobilizing immune activation in response to microbial invasion and promoting wound healing to repair damaged tissue. However, failure to resolve macrophage activation can lead to chronic inflammation and fibrosis, and ultimately to pathology. Activated macrophages have been implicated in the pathogenesis of systemic sclerosis (SSc), although the triggers that induce immune activation in SSc and the signaling pathways that underlie aberrant macrophage activation remain unknown.
Recent Findings
Macrophages are implicated in fibrotic activation in SSc. Targeted therapeutic interventions directed against SSc macrophages may ameliorate inflammation and fibrosis.
Summary
While current studies have begun to elucidate the role of macrophages in disease initiation and progression, further work is needed to address macrophage subset heterogeneity within and among SSc end-target tissues to determine the disparate functions mediated by these subsets and to identify additional targets for therapeutic intervention.

Similar content being viewed by others
Abbreviations
- SSc:
-
Systemic sclerosis
- ECM:
-
Extracellular matrix
- VEGF:
-
Vascular endothelial growth factor
- pDCs:
-
Plasmacytoid dendritic cells
- MMF:
-
Mycophenolate mofetil
- PFD:
-
Pirfenidone
- IPF:
-
Idiopathic pulmonary fibrosis
- NTD:
-
Nintedanib
- PDGF:
-
Platelet-derived growth factor
- FGF:
-
Fibroblast growth factor
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Assassi S, Swindell WR, Wu M, Tan FD, Khanna D, Furst DE, et al. Dissecting the heterogeneity of skin gene expression patterns in systemic sclerosis. Arthritis Rheum. 2015;67:3016–26.
Mahoney JM, Taroni J, Martyanov V, Wood TA, Greene CS, Pioli PA, et al. Systems level analysis of systemic sclerosis shows a network of immune and profibrotic pathways connected with genetic polymorphisms. PLoS Comput Biol. 2015;11:e1004005.
• Taroni JN, Greene CS, Martyanov V, Wood TA, Christmann RB, Farber HW, et al. A novel multi-network approach reveals tissue-specific cellular modulators of fibrosis in systemic sclerosis. Genome Med. 2017;9:27 A meta-analysis combining multiple gene expression datasets from different SSc-affected tissues, identifying a cluster of genes associated with alternatively activated macrophages.
•• Hinchcliff M, Toledo DM, Taroni JN, Wood TA, Franks JM, Ball MS, et al. Mycophenolate mofetil treatment of systemic sclerosis reduces myeloid cell numbers and attenuates the inflammatory gene signature in skin. J Invest Dermatol. 2018;138:1301–10 This study focuses on the molecular effects of long-term MMF therapy on the macrophages in SSc patient skin.
Milano A, Pendergrass SA, Sargent JL, George LK, McCalmont TH, Connolly MK, et al. Molecular subsets in the gene expression signatures of scleroderma skin. PLoS One. 2008;3:e2696.
Pendergrass SA, Lemaire R, Francis IP, Mahoney JM, Lafyatis R, Whitfield ML. Intrinsic gene expression subsets of diffuse cutaneous systemic sclerosis are stable in serial skin biopsies. J Invest Dermatol. 2012;132:1363–73.
Hinchcliff M, Huang CC, Wood TA, Matthew Mahoney J, Martyanov V, Bhattacharyya S, et al. Molecular signatures in skin associated with clinical improvement during mycophenolate treatment in systemic sclerosis. J Invest Dermatol. 2013;133:1979–89.
Johnson ME, Pioli PA, Whitfield ML. Gene expression profiling offers insights into the role of innate immune signaling in SSc. Semin Immunopathol. 2015;37:501–9.
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86.
Christmann RB, Sampaio-Barros P, Stifano G, Borges CL, de Carvalho CR, Kairalla R, et al. Association of Interferon- and transforming growth factor beta-regulated genes and macrophage activation with systemic sclerosis-related progressive lung fibrosis. Arthritis Rheum. 2014;66:714–25.
Higashi-Kuwata N, Jinnin M, Makino T, Fukushima S, Inoue Y, Muchemwa FC, et al. Characterization of monocyte/macrophage subsets in the skin and peripheral blood derived from patients with systemic sclerosis. Arthritis Res Ther. 2010;12:R128.
•• Soldano S, Trombetta AC, Contini P, Tomatis V, Ruaro B, Brizzolara R, et al. Increase in circulating cells coexpressing M1 and M2 macrophage surface markers in patients with systemic sclerosis. Ann Rheum Dis. 2018;77:1842–+ This study finds that circulating monocytes from the blood of patients with SSc have a mixed M1/M2 phenotype.
Trombetta AC, Soldano S, Contini P, Tomatis V, Ruaro B, Paolino S, et al. A circulating cell population showing both M1 and M2 monocyte/macrophage surface markers characterizes systemic sclerosis patients with lung involvement. Respir Res. 2018;19:186.
Lescoat A, Ballerie A, Jouneau S, Fardel O, Vernhet L, Jego P, Lecureur V. 2018. M1/M2 polarisation state of M-CSF blood-derived macrophages in systemic sclerosis. Ann Rheum Dis, annrheumdis-2018-214333.
Ushach I, Zlotnik A. Biological role of granulocyte macrophage colony-stimulating factor (GM-CSF) and macrophage colony-stimulating factor (M-CSF) on cells of the myeloid lineage. J Leukoc Biol. 2016;100:481–9.
Mathai SK, Gulati M, Peng X, Russell TR, Shaw AC, Rubinowitz AN, et al. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Investig. 2010;90:812–23.
• Moreno-Moral A, Bagnati M, Koturan S, Ko JH, Fonseca C, Harmston N, et al. Changes in macrophage transcriptome associate with systemic sclerosis and mediate GSDMA contribution to disease risk. Ann Rheum Dis. 2018;77:596–601 This study focuses on the gene expression of monocyte-derived macrophages from patients with SSc and healthy controls, and performs an eQTL analysis with previous GWAS findings and reports that GSDMA gene upregulation was cis-regulated by SNP rs3859192.
Terao C, Kawaguchi T, Dieude P, Varga J, Kuwana M, Hudson M, et al. Transethnic meta-analysis identifies GSDMA and PRDM1 as susceptibility genes to systemic sclerosis. Ann Rheum Dis. 2017;76:1150–8.
Lei M, Bai X, Yang T, Lai X, Qiu W, Yang L, et al. Gsdma3 is a new factor needed for TNF-alpha-mediated apoptosis signal pathway in mouse skin keratinocytes. Histochem Cell Biol. 2012;138:385–96.
Farina A, Peruzzi G, Lacconi V, Lenna S, Quarta S, Rosato E, et al. Epstein-Barr virus lytic infection promotes activation of Toll-like receptor 8 innate immune response in systemic sclerosis monocytes. Arthritis Res Ther. 2017;19:39.
Ah Kioon MD, Tripodo C, Fernandez D, Kirou KA, Spiera RF, Crow MK, et al. Plasmacytoid dendritic cells promote systemic sclerosis with a key role for TLR8. Sci Transl Med. 2018;10:eaam8458.
Arron ST, Dimon MT, Li Z, Johnson ME, Wood TA, Feeney L, et al. High Rhodotorula sequences in skin transcriptome of patients with diffuse systemic sclerosis. J Invest Dermatol. 2014;134:2138–45.
Johnson ME, Franks JM, Cai G, Mehta BK, Wood TA, Archambault K, et al. Microbiome dysbiosis is associated with disease duration and increased inflammatory gene expression in systemic sclerosis skin. Arthritis Res Ther. 2019;21:49.
O'Reilly S. Pound the alarm: danger signals in rheumatic diseases. Clin Sci (Lond). 2015;128:297–305.
O'Reilly S, van Laar JM. Targeting the TLR4-MD2 axis in systemic sclerosis. Nat Rev Rheumatol. 2018;14:564–6.
Bhattacharyya S, Wang W, Morales-Nebreda L, Feng G, Wu M, Zhou X, et al. Tenascin-C drives persistence of organ fibrosis. Nat Commun. 2016;7:11703.
Ogawa F, Shimizu K, Hara T, Muroi E, Hasegawa M, Takehara K, et al. Serum levels of heat shock protein 70, a biomarker of cellular stress, are elevated in patients with systemic sclerosis: association with fibrosis and vascular damage. Clin Exp Rheumatol. 2008;26:659–62.
Tomcik M, Zerr P, Pitkowski J, Palumbo-Zerr K, Avouac J, Distler O, et al. Heat shock protein 90 (Hsp90) inhibition targets canonical TGF-beta signalling to prevent fibrosis. Ann Rheum Dis. 2014;73:1215–22.
Saito K, Kukita K, Kutomi G, Okuya K, Asanuma H, Tabeya T, et al. Heat shock protein 90 associates with Toll-like receptors 7/9 and mediates self-nucleic acid recognition in SLE. Eur J Immunol. 2015;45:2028–41.
Vabulas RM, Ahmad-Nejad P, Ghose S, Kirschning CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–12.
Feng Y, Ren J, Gui Y, Wei W, Shu B, Lu Q, et al. Wnt/beta-catenin-promoted macrophage alternative activation contributes to kidney fibrosis. J Am Soc Nephrol. 2018;29:182–93.
Sennello JA, Misharin AV, Flozak AS, Berdnikovs S, Cheresh P, Varga J, et al. Lrp5/beta-catenin signaling controls lung macrophage differentiation and inhibits resolution of fibrosis. Am J Respir Cell Mol Biol. 2017;56:191–201.
Wei J, Fang F, Lam AP, Sargent JL, Hamburg E, Hinchcliff ME, et al. Wnt/beta-catenin signaling is hyperactivated in systemic sclerosis and induces Smad-dependent fibrotic responses in mesenchymal cells. Arthritis Rheum. 2012;64:2734–45.
• Lafyatis R, Mantero JC, Gordon J, Kishore N, Carns M, Dittrich H, et al. Inhibition of beta-catenin signaling in the skin rescues cutaneous adipogenesis in systemic sclerosis: a randomized, double-blind, placebo-controlled trial of C-82. J Investig Dermatol. 2017;137:2473–83 This study showed that the inhibition of β-catenin signaling with a topical inhibitor showed therapeutic effects to SSc-affected skin.
Tao B, Jin W, Xu JQ, Liang ZY, Yao JL, Zhang Y, et al. Myeloid-specific disruption of tyrosine phosphatase Shp2 promotes alternative activation of macrophages and predisposes mice to pulmonary fibrosis. J Immunol. 2014;193:2801–11.
Weng SY, Wang XY, Vijayan S, Tang YL, Kim YO, Padberg K, et al. IL-4 receptor alpha signaling through macrophages differentially regulates liver fibrosis progression and reversal. Ebiomedicine. 2018;29:92–103.
Dantas AT, de Almeida AR, Sampaio MCPD, Cordeiro MF, de Oliveira PSS, Mariz HD, et al. Different profile of cytokine production in patients with systemic sclerosis and association with clinical manifestations. Immunol Lett. 2018;198:12–6.
Needleman BW, Wigley FM, Stair RW. Interleukin-1, interleukin-2, interleukin-4, interleukin-6, tumor-necrosis-factor-alpha, and interferon-gamma levels in sera from patients with scleroderma. Arthritis Rheum. 1992;35:67–72.
Rebe C, Vegran F, Berger H, Ghiringhelli F. STAT3 activation: a key factor in tumor immunoescape. JAKSTAT. 2013;2:e23010.
Chakraborty D, Sumova B, Mallano T, Chen CW, Distler A, Bergmann C, et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun. 2017;8:1130.
Papaioannou I, Xu S, Denton CP, Abraham DJ, Ponticos M. STAT3 controls COL1A2 enhancer activation cooperatively with JunB, regulates type I collagen synthesis posttranscriptionally, and is essential for lung myofibroblast differentiation. Mol Biol Cell. 2018;29:84–95.
Pedroza M, To S, Assassi S, Wu M, Tweardy D, Agarwal SK. Role of STAT3 in skin fibrosis and transforming growth factor beta signalling. Rheumatology (Oxford). 2018;57:1838–50.
Morris E, Chrobak I, Bujor A, Hant F, Mummery C, Ten Dijke P, et al. Endoglin promotes TGF-beta/Smad1 signaling in scleroderma fibroblasts. J Cell Physiol. 2011;226:3340–8.
Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–62.
Nacu N, Luzina IG, Highsmith K, Lockatell V, Pochetuhen K, Cooper ZA, et al. Macrophages produce TGF-beta-induced (beta-ig-h3) following ingestion of apoptotic cells and regulate MMP14 levels and collagen turnover in fibroblasts. J Immunol. 2008;180:5036–44.
Ballerie A, Lescoat A, Augagneur Y, Lelong M, Morzadec C, Cazalets C, et al. Efferocytosis capacities of blood monocyte-derived macrophages in systemic sclerosis. Immunol Cell Biol. 2019;97:340–7.
Rice LM, Padilla CM, McLaughlin SR, Mathes A, Ziemek J, Goummih S, et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest. 2015;125:2795–807.
van der Heijden C, Noz MP, Joosten LAB, Netea MG, Riksen NP, Keating ST. Epigenetics and trained immunity. Antioxid Redox Signal. 2018;29:1023–40.
Arts RJW, Joosten LAB, Netea MG. The potential role of trained immunity in autoimmune and autoinflammatory disorders. Front Immunol. 2018;9:298.
Ciechomska M, O'Reilly S, Przyborski S, Oakley F, Bogunia-Kubik K, van Laar JM. Histone demethylation and toll-like receptor 8-dependent cross-talk in monocytes promotes transdifferentiation of fibroblasts in systemic sclerosis via Fra-2. Arthritis Rheum. 2016;68:1493–504.
•• van der Kroef M, Castellucci M, Mokry M, Cossu M, Garonzi M, Bossini-Castillo LM, et al. Histone modifications underlie monocyte dysregulation in patients with systemic sclerosis, underlining the treatment potential of epigenetic targeting. Ann Rheum Dis. 2019;78:529–38 This study identified altered global methylation marks in monocytes from patients with SSc when compared with healthy control monocytes.
• Ramos PS, Zimmerman KD, Haddad S, Langefeld CD, Medsger TA Jr, Feghali-Bostwick CA. Integrative analysis of DNA methylation in discordant twins unveils distinct architectures of systemic sclerosis subsets. Clin Epigenetics. 2019;11:58 Recent study that found an enrichment of methylated cytosines in regulatory regions in myeloid cells.
Trachtman H, Fervenza FC, Gipson DS, Heering P, Jayne DR, Peters H, et al. A phase 1, single-dose study of fresolimumab, an anti-TGF-beta antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int. 2011;79:1236–43.
Vincenti F, Fervenza FC, Campbell KN, Diaz M, Gesualdo L, Nelson P, et al. A phase 2, double-blind, placebo-controlled, randomized study of fresolimumab in patients with steroid-resistant primary focal segmental glomerulosclerosis. Kidney Int Rep. 2017;2:800–10.
He X, Theegarten D, Guzman J, Costabel U, Bonella F. Effect of pirfenidone (PFD) on cytokine/chemokine release from alveolar macrophages (AMs) in interstitial lung diseases (ILD): preliminary results. Eur Respir J. 2013;42:P2334.
Toda M, Mizuguchi S, Minamiyama Y, Yamamoto-Oka H, Aota T, Kubo S, et al. Pirfenidone suppresses polarization to M2 phenotype macrophages and the fibrogenic activity of rat lung fibroblasts. J Clin Biochem Nutr. 2018;63:58–65.
•• Du J, Paz K, Flynn R, Vulic A, Robinson TM, Lineburg KE, et al. Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF-beta production. Blood. 2017;129:2570–80 This study finds that in the cGVHD mouse model of fibrosis and SSc, pirfenidone decreased macrophage infiltration, reduced macrophage chemotaxis toward CCL2, and ameliorated symptoms of fibrosis.
• Khanna D, Albera C, Fischer A, Khalidi N, Raghu G, Chung L, et al. An open-label, phase II study of the safety and tolerability of pirfenidone in patients with scleroderma-associated interstitial lung disease: the LOTUSS trial. J Rheumatol. 2016;43:1672–9 Phase II trial to study the safety and tolerability of pirfenidone in SSc patients with interstitial lung disease.
Xiao H, Zhang GF, Liao XP, Li XJ, Zhang J, Lin H, et al. Anti-fibrotic effects of pirfenidone by interference with the hedgehog signalling pathway in patients with systemic sclerosis-associated interstitial lung disease. Int J Rheum Dis. 2018;21:477–86.
Seki E. HEDGEHOG signal in hepatocytes mediates macrophage recruitment: a new mechanism and potential therapeutic target for fatty liver disease. Hepatology. 2016;63:1071–3.
Pereira TA, Xie G, Choi SS, Syn WK, Voieta I, Lu J, et al. Macrophage-derived hedgehog ligands promotes fibrogenic and angiogenic responses in human schistosomiasis mansoni. Liver Int. 2013;33:149–61.
•• Huang J, Maier C, Zhang Y, Soare A, Dees C, Beyer C, et al. Nintedanib inhibits macrophage activation and ameliorates vascular and fibrotic manifestations in the Fra2 mouse model of systemic sclerosis. Ann Rheum Dis. 2017;76:1941–8 This study shows that nintedanib reduces macrophage activation and ameliorates symptoms of fibrosis in the Fra-2 mouse model of SSc.
Maurer B, Distler JH, Distler O. The Fra-2 transgenic mouse model of systemic sclerosis. Vasc Pharmacol. 2013;58:194–201.
Bellamri N, Morzadec C, Lecureur V, Joannes A, Wollin L, Jouneau S, et al. Effects of Nintedanib on the M1 and M2a polarization of human macrophages. Eur Respir J. 2018;52:PA5250.
Distler O, Brown KK, Distler JHW, Assassi S, Maher TM, Cottin V, et al. Design of a randomised, placebo-controlled clinical trial of nintedanib in patients with systemic sclerosis-associated interstitial lung disease (SENSCIS). Clin Exp Rheumatol. 2017;35(Suppl 106):75–81.
Elhai M, Meunier M, Matucci-Cerinic M, Maurer B, Riemekasten G, Leturcq T, et al. Outcomes of patients with systemic sclerosis-associated polyarthritis and myopathy treated with tocilizumab or abatacept: a EUSTAR observational study. Ann Rheum Dis. 2013;72:1217–20.
Shima Y, Kuwahara Y, Murota H, Kitaba S, Kawai M, Hirano T, et al. The skin of patients with systemic sclerosis softened during the treatment with anti-IL-6 receptor antibody tocilizumab. Rheumatology (Oxford). 2010;49:2408–12.
• Khanna D, Denton CP, Lin CJF, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomised controlled trial (faSScinate). Ann Rheum Dis. 2018;77:212–20 Phase II study of tocilizumab treatment for SSc, finding that expression of macrophage activation markers, like CCL18, was reduced after treatment, and clinical increases in lung function were observed.
Mauer J, Denson JL, Bruning JC. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2015;36:92–101.
Funding
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R56-AR0639835 and R03-AR068097 (PAP) and by a grant from the Scleroderma Foundation (PAP). DMT received support from the National Institutes of Health p50 Specialized Center Grant, Research Diversity Supplement (3P50AR060780 - 07W1) and the John H. Copenhaver, Jr. and William H. Thomas, MD 1952 Junior Fellowship from Dartmouth Graduate Studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ms. Toledo reports grants from NIH and from Dartmouth Graduate Studies, during the conduct of the study.
Dr. Pioli reports grants from Celdara Medical, LLC, grants from NIH/NIAMS, and grants from Scleroderma Foundation, during the conduct of the study; in addition, Dr. Pioli has a patent Cellular Based Therapies Targeting Disease-Associated Molecular Mediators of Fibrotic, Inflammatory, and Autoimmune Conditions pending.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Scleroderma
Rights and permissions
About this article
Cite this article
Toledo, D.M., Pioli, P.A. Macrophages in Systemic Sclerosis: Novel Insights and Therapeutic Implications. Curr Rheumatol Rep 21, 31 (2019). https://doi.org/10.1007/s11926-019-0831-z
Published:
DOI: https://doi.org/10.1007/s11926-019-0831-z