Abstract
Purpose of the Review
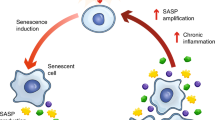
Senescent cells have the capacity to both effect and limit fibrosis. Senotherapeutics target senescent cells to improve aging conditions. Here, we review the contexts in which senescent cells mediate wound healing and fibrotic pathology and the potential utility of senotherapeutic drugs for treatment of fibrotic disease.
Recent Findings
Multi-action and temporal considerations influence deleterious versus beneficial actions of senescent cells. Acutely generated senescent cells can limit proliferation, and the senescence-associated secretory phenotype (SASP) contains factors that can facilitate tissue repair. Long-lived senescent cells that evade clearance or are generated outside of programmed remodeling can deplete the progenitor pool to exhaust regenerative capacity and through the SASP, stimulate continual activation, leading to disorganized tissue architecture, fibrotic damage, sterile inflammation, and induction of bystander senescence.
Summary
Senescent cells contribute to fibrotic pathogenesis in multiple tissues, including the liver, kidney, and lung. Senotherapeutics may be a viable strategy for treatment of a range of fibrotic conditions.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–40. https://doi.org/10.1038/nm.2807.
Schafer MJ, Miller JD, LeBrasseur NK. Cellular senescence: implications for metabolic disease. Mol Cell Endocrinol. 2017;455:93–102. https://doi.org/10.1016/j.mce.2016.08.047.
• Munoz-Espin D, Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15(7):482–96. This review discusses the evidence for benefical and deleterious roles of senescent cells in a range of diseases. https://doi.org/10.1038/nrm3823.
Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, al-Regaiey K, Su L, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Investig. 2004;114(9):1299–307. https://doi.org/10.1172/JCI22475.
Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–22. https://doi.org/10.1016/j.cell.2005.02.003.
Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–40. https://doi.org/10.1038/nrm2233.
Alcorta DA, Xiong Y, Phelps D, Hannon G, Beach D, Barrett JC. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci U S A. 1996;93(24):13742–7. https://doi.org/10.1073/pnas.93.24.13742.
Beausejour CM, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J. 2003;22(16):4212–22. https://doi.org/10.1093/emboj/cdg417.
Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer. 2009;9(2):81–94. https://doi.org/10.1038/nrc2560.
Korolchuk VI, Miwa S, Carroll B, von Zglinicki T. Mitochondria in cell senescence: is mitophagy the weakest link? eBioMedicine. 2017;21:7–13. https://doi.org/10.1016/j.ebiom.2017.03.020.
Zou Y, Sfeir A, Gryaznov SM, Shay JW, Wright WE. Does a sentinel or a subset of short telomeres determine replicative senescence? Mol Biol Cell. 2004;15(8):3709–18. https://doi.org/10.1091/mbc.E04-03-0207.
d'Adda di Fagagna F, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426(6963):194–8. https://doi.org/10.1038/nature02118.
Hewitt G, Jurk D, Marques FDM, Correia-Melo C, Hardy T, Gackowska A, et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun. 2012;3:708. https://doi.org/10.1038/ncomms1708.
Adams PD. Remodeling of chromatin structure in senescent cells and its potential impact on tumor suppression and aging. Gene. 2007;397(1-2):84–93. https://doi.org/10.1016/j.gene.2007.04.020.
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A. 1995;92(20):9363–7. https://doi.org/10.1073/pnas.92.20.9363.
Bayreuther K, Rodemann HP, Hommel R, Dittmann K, Albiez M, Francz PI. Human skin fibroblasts in vitro differentiate along a terminal cell lineage. Proc Natl Acad Sci U S A. 1988;85(14):5112–6. https://doi.org/10.1073/pnas.85.14.5112.
Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117(3):524–9. https://doi.org/10.1172/JCI31487.
Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43(1):146–55. https://doi.org/10.1016/j.jbiomech.2009.09.020.
Kendall RT, Feghali-Bostwick CA. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014;5:123. https://doi.org/10.3389/fphar.2014.00123.
Tschumperlin DJ. Matrix, mesenchyme, and mechanotransduction. Ann Am Thorac Soc. 2015;12(Suppl 1):S24–9. https://doi.org/10.1513/AnnalsATS.201407-320MG.
Tschumperlin DJ, Liu F, Tager AM. Biomechanical regulation of mesenchymal cell function. Curr Opin Rheumatol. 2013;25(1):92–100. https://doi.org/10.1097/BOR.0b013e32835b13cd.
• Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31(6):722–33. This study demonstrates a beneficial role for senscence in optimal skin wound healing using a novel suicide-gene transgenic mouse model in which senescent cells can be monitored and deleted. https://doi.org/10.1016/j.devcel.2014.11.012.
Jun JI, Lau LF. Cellular senescence controls fibrosis in wound healing. Aging (Albany NY). 2010;2(9):627–31. https://doi.org/10.18632/aging.100201.
Lehmann M, et al.. Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo. Eur Respir J. 2017;50. https://doi.org/10.1183/13993003.02367-2016
• Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun. 2017;8:14532. This study demonstrates a detrimental role for senscence in fibrotic lung disease, which is ameliorated by transgenic or senolytic clearance of senescent cells. https://doi.org/10.1038/ncomms14532.
Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134(4):657–67. https://doi.org/10.1016/j.cell.2008.06.049.
Hecker L, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6:231ra247. https://doi.org/10.1126/scitranslmed.3008182.
Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5(1):99–118. https://doi.org/10.1146/annurev-pathol-121808-102144.
Ozcan S, et al. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging (Albany NY). 2016;8(7):1316–29. https://doi.org/10.18632/aging.100971.
Maciel-Baron LA, et al. Senescence associated secretory phenotype profile from primary lung mice fibroblasts depends on the senescence induction stimuli. Age (Dordr). 2016;38(1):26. https://doi.org/10.1007/s11357-016-9886-1.
Perez-Mancera PA, Young AR, Narita M. Inside and out: the activities of senescence in cancer. Nat Rev Cancer. 2014;14(8):547–58. https://doi.org/10.1038/nrc3773.
Ashcroft GS, Horan MA, Ferguson MW. Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab Investig. 1998;78(1):47–58.
Waaijer ME, et al. The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell. 2012;11(4):722–5. https://doi.org/10.1111/j.1474-9726.2012.00837.x.
Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter-Kochanek K, Jansen-Durr P, et al. p16INK4A is a robust in vivo biomarker of cellular aging in human skin. Aging Cell. 2006;5(5):379–89. https://doi.org/10.1111/j.1474-9726.2006.00231.x.
Adamus J, Aho S, Meldrum H, Bosko C, Lee JM. p16INK4A influences the aging phenotype in the living skin equivalent. J Investig Dermatol. 2014;134(4):1131–3. https://doi.org/10.1038/jid.2013.468.
Velarde MC, Flynn JM, Day NU, Melov S, Campisi J. Mitochondrial oxidative stress caused by Sod2 deficiency promotes cellular senescence and aging phenotypes in the skin. Aging (Albany NY). 2012;4(1):3–12. https://doi.org/10.18632/aging.100423.
Velarde MC, Demaria M, Melov S, Campisi J. Pleiotropic age-dependent effects of mitochondrial dysfunction on epidermal stem cells. Proc Natl Acad Sci U S A. 2015;112(33):10407–12. https://doi.org/10.1073/pnas.1505675112.
Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12(7):676–85. https://doi.org/10.1038/ncb2070.
Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J, et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002;16(9):935–42. https://doi.org/10.1096/fj.01-0977com.
Paradis V, Youssef N, Dargère D, Bâ N, Bonvoust F, Deschatrette J, et al. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol. 2001;32(3):327–32. https://doi.org/10.1053/hupa.2001.22747.
Aravinthan A, Mells G, Allison M, Leathart J, Kotronen A, Yki-Jarvinen H, et al. Gene polymorphisms of cellular senescence marker p21 and disease progression in non-alcohol-related fatty liver disease. Cell Cycle. 2014;13(9):1489–94. https://doi.org/10.4161/cc.28471.
Lunz JG 3rd, et al. Replicative senescence of biliary epithelial cells precedes bile duct loss in chronic liver allograft rejection: increased expression of p21(WAF1/Cip1) as a disease marker and the influence of immunosuppressive drugs. Am J Pathol. 2001;158(4):1379–90. https://doi.org/10.1016/S0002-9440(10)64089-8.
Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y. Autophagy may precede cellular senescence of bile ductular cells in ductular reaction in primary biliary cirrhosis. Dig Dis Sci. 2012;57(3):660–6. https://doi.org/10.1007/s10620-011-1929-y.
Borkham-Kamphorst E, Schaffrath C, van de Leur E, Haas U, Tihaa L, Meurer SK, et al. The anti-fibrotic effects of CCN1/CYR61 in primary portal myofibroblasts are mediated through induction of reactive oxygen species resulting in cellular senescence, apoptosis and attenuated TGF-beta signaling. Biochim Biophys Acta. 2014;1843(5):902–14. https://doi.org/10.1016/j.bbamcr.2014.01.023.
Kim KH, Chen CC, Monzon RI, Lau LF. Matricellular protein CCN1 promotes regression of liver fibrosis through induction of cellular senescence in hepatic myofibroblasts. Mol Cell Biol. 2013;33(10):2078–90. https://doi.org/10.1128/MCB.00049-13.
Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, et al. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56(3):1150–9. https://doi.org/10.1002/hep.25744.
Sagiv A, Burton DGA, Moshayev Z, Vadai E, Wensveen F, Ben-Dor S, et al. NKG2D ligands mediate immunosurveillance of senescent cells. Aging (Albany NY). 2016;8(2):328–44. https://doi.org/10.18632/aging.100897.
Bataller R, Brenner DA. Liver fibrosis. J Clin Investig. 2005;115(2):209–18. https://doi.org/10.1172/JCI24282.
Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, et al. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8:15691. https://doi.org/10.1038/ncomms15691.
Demirci G, Nashan B, Pichlmayr R. Fibrosis in chronic rejection of human liver allografts: expression patterns of transforming growth factor-TGFbeta1 and TGF-beta3. Transplantation. 1996;62(12):1776–83. https://doi.org/10.1097/00007890-199612270-00016.
Ding G, Franki N, Kapasi AA, Reddy K, Gibbons N, Singhal PC. Tubular cell senescence and expression of TGF-beta1 and p21(WAF1/CIP1) in tubulointerstitial fibrosis of aging rats. Exp Mol Pathol. 2001;70(1):43–53. https://doi.org/10.1006/exmp.2000.2346.
Liu J, Yang JR, He YN, Cai GY, Zhang JG, Lin LR, et al. Accelerated senescence of renal tubular epithelial cells is associated with disease progression of patients with immunoglobulin A (IgA) nephropathy. Transl Res. 2012;159(6):454–63. https://doi.org/10.1016/j.trsl.2011.11.008.
Verzola D, Gandolfo MT, Gaetani G, Ferraris A, Mangerini R, Ferrario F, et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am J Physiol Renal Physiol. 2008;295(5):F1563–73. https://doi.org/10.1152/ajprenal.90302.2008.
Wolstein JM, Lee DH, Michaud J, Buot V, Stefanchik B, Plotkin MD. INK4a knockout mice exhibit increased fibrosis under normal conditions and in response to unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2010;299(6):F1486–95. https://doi.org/10.1152/ajprenal.00378.2010.
Clements ME, Chaber CJ, Ledbetter SR, Zuk A. Increased cellular senescence and vascular rarefaction exacerbate the progression of kidney fibrosis in aged mice following transient ischemic injury. PLoS One. 2013;8(8):e70464. https://doi.org/10.1371/journal.pone.0070464.
Braun H, Schmidt BMW, Raiss M, Baisantry A, Mircea-Constantin D, Wang S, et al. Cellular senescence limits regenerative capacity and allograft survival. J Am Soc Nephrol. 2012;23(9):1467–73. https://doi.org/10.1681/ASN.2011100967.
Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, Fridman E, et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol. 2009;20(8):1754–64. https://doi.org/10.1681/ASN.2008111204.
Joosten SA, van Ham V, Nolan CE, Borrias MC, Jardine AG, Shiels PG, et al. Telomere shortening and cellular senescence in a model of chronic renal allograft rejection. Am J Pathol. 2003;162(4):1305–12. https://doi.org/10.1016/S0002-9440(10)63926-0.
Chkhotua AB, Altimari A, Gabusi E, D’Errico A, Yakubovich M, Vienken J, et al. Increased expression of P21((WAF1/CIP1)) CDKI gene in chronic allograft nephropathy correlating with the number of acute rejection episodes. Transplant Proc. 2003;35(2):655–8. https://doi.org/10.1016/S0041-1345(03)00025-3.
Melk A, Schmidt BM, Vongwiwatana A, Rayner DC, Halloran PF. Increased expression of senescence-associated cell cycle inhibitor p16INK4a in deteriorating renal transplants and diseased native kidney. Am J Transplant. 2005;5(6):1375–82. https://doi.org/10.1111/j.1600-6143.2005.00846.x.
McGlynn LM, Stevenson K, Lamb K, Zino S, Brown M, Prina A, et al. Cellular senescence in pretransplant renal biopsies predicts postoperative organ function. Aging Cell. 2009;8(1):45–51. https://doi.org/10.1111/j.1474-9726.2008.00447.x.
Chkhotua AB, Abendroth D, Froeba G, Schelzig H. Up-regulation of cell cycle regulatory genes after renal ischemia/reperfusion: differential expression of p16(INK4a), p21(WAF1/CIP1) and p27(Kip1) cyclin-dependent kinase inhibitor genes depending on reperfusion time. Transpl Int. 2006;19(1):72–7. https://doi.org/10.1111/j.1432-2277.2005.00227.x.
Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16(5):535–43. https://doi.org/10.1038/nm.2144.
Muller KC, et al. Lung fibroblasts from patients with emphysema show markers of senescence in vitro. Respir Res. 2006;7(1):32. https://doi.org/10.1186/1465-9921-7-32.
Kuwano K, Kunitake R, Kawasaki M, Nomoto Y, Hagimoto N, Nakanishi Y, et al. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1996;154(2):477–83. https://doi.org/10.1164/ajrccm.154.2.8756825.
Lomas NJ, Watts KL, Akram KM, Forsyth NR, Spiteri MA. Idiopathic pulmonary fibrosis: immunohistochemical analysis provides fresh insights into lung tissue remodelling with implications for novel prognostic markers. Int J Clin Exp Pathol. 2012;5(1):58–71.
Alvarez D, et al.. IPF lung fibroblasts have a senescent phenotype. Am J Physiol Lung Cell Mol Physiol. 2017;ajplung 00220 02017. https://doi.org/10.1152/ajplung.00220.2017.
Tsuji T, Aoshiba K, Nagai A. Alveolar cell senescence in patients with pulmonary emphysema. Am J Respir Crit Care Med. 2006;174(8):886–93. https://doi.org/10.1164/rccm.200509-1374OC.
Disayabutr S, Kim EK, Cha SI, Green G, Naikawadi RP, Jones KD, et al. miR-34 miRNAs regulate cellular senescence in type II alveolar epithelial cells of patients with idiopathic pulmonary fibrosis. PLoS One. 2016;11(6):e0158367. https://doi.org/10.1371/journal.pone.0158367.
Fischer BM, Wong JK, Degan S, Kummarapurugu AB, Zheng S, Haridass P, et al. Increased expression of senescence markers in cystic fibrosis airways. Am J Physiol Lung Cell Mol Physiol. 2013;304(6):L394–400. https://doi.org/10.1152/ajplung.00091.2012.
Yanai H, Shteinberg A, Porat Z, Budovsky A, Braiman A, Zeische R, et al. Cellular senescence-like features of lung fibroblasts derived from idiopathic pulmonary fibrosis patients. Aging (Albany NY). 2015;7(9):664–72. https://doi.org/10.18632/aging.100807.
Holz O, Zühlke I, Jaksztat E, Müller KC, Welker L, Nakashima M, et al. Lung fibroblasts from patients with emphysema show a reduced proliferation rate in culture. Eur Respir J. 2004;24(4):575–9. https://doi.org/10.1183/09031936.04.00143703.
Izbicki G, Segel MJ, Christensen TG, Conner MW, Breuer R. Time course of bleomycin-induced lung fibrosis. Int J Exp Pathol. 2002;83(3):111–9. https://doi.org/10.1046/j.1365-2613.2002.00220.x.
Aoshiba K, Tsuji T, Nagai A. Bleomycin induces cellular senescence in alveolar epithelial cells. Eur Respir J. 2003;22(3):436–43. https://doi.org/10.1183/09031936.03.00011903.
Aoshiba K, Tsuji T, Kameyama S, Itoh M, Semba S, Yamaguchi K, et al. Senescence-associated secretory phenotype in a mouse model of bleomycin-induced lung injury. Exp Toxicol Pathol. 2013;65(7-8):1053–62. https://doi.org/10.1016/j.etp.2013.04.001.
Hashimoto M, et al. Elimination of p19ARF-expressing cells enhances pulmonary function in mice. JCI Insight 1. https://doi.org/10.1172/jci.insight.87732.
Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, et al. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208(7):1459–71. https://doi.org/10.1084/jem.20102510.
Li Y, et al.. Hyaluronan synthase 2 regulates fibroblast senescence in pulmonary fibrosis. Matrix Biol. 2016. https://doi.org/10.1016/j.matbio.2016.03.004.
Roos CM, Hagler M, Zhang B, Oehler EA, Arghami A, Miller JD. Transcriptional and phenotypic changes in aorta and aortic valve with aging and MnSOD deficiency in mice. Am J Physiol Heart Circ Physiol. 2013;305(10):H1428–39. https://doi.org/10.1152/ajpheart.00735.2012.
Vasile E, Tomita Y, Brown LF, Kocher O, Dvorak HF. Differential expression of thymosin beta-10 by early passage and senescent vascular endothelium is modulated by VPF/VEGF: evidence for senescent endothelial cells in vivo at sites of atherosclerosis. FASEB J. 2001;15(2):458–66. https://doi.org/10.1096/fj.00-0051com.
Minamino T, Yoshida T, Tateno K, Miyauchi H, Zou Y, Toko H, et al. Ras induces vascular smooth muscle cell senescence and inflammation in human atherosclerosis. Circulation. 2003;108(18):2264–9. https://doi.org/10.1161/01.CIR.0000093274.82929.22.
Roos CM, et al.. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016. https://doi.org/10.1111/acel.12458.
Zhu F, Li Y, Zhang J, Piao C, Liu T, Li HH, et al. Senescent cardiac fibroblast is critical for cardiac fibrosis after myocardial infarction. PLoS One. 2013;8(9):e74535. https://doi.org/10.1371/journal.pone.0074535.
Jaster R, Emmrich J. Crucial role of fibrogenesis in pancreatic diseases. Best Pract Res Clin Gastroenterol. 2008;22(1):17–29. https://doi.org/10.1016/j.bpg.2007.10.004.
Fitzner B, Müller S, Walther M, Fischer M, Engelmann R, Müller-Hilke B, et al. Senescence determines the fate of activated rat pancreatic stellate cells. J Cell Mol Med. 2012;16(11):2620–30. https://doi.org/10.1111/j.1582-4934.2012.01573.x.
Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138(17):3639–46. https://doi.org/10.1242/dev.067595.
Sousa-Victor P, Gutarra S, García-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506(7488):316–21. https://doi.org/10.1038/nature13013.
Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530(7589):184–9. https://doi.org/10.1038/nature16932.
Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479(7372):232–6. https://doi.org/10.1038/nature10600.
Schafer MJ, et al.. Exercise prevents diet-induced cellular senescence in adipose tissue. Diabetes. 2016. https://doi.org/10.2337/db15-0291.
Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23(9):1072–9. https://doi.org/10.1038/nm.4385.
•• Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–58. This study describes the first discovery of senolytic drugs. https://doi.org/10.1111/acel.12344.
Condorelli F, Genazzani AA. Dasatinib is it all in the dose? BioDrugs. 2010;24(3):157–63. https://doi.org/10.2165/11535870-000000000-00000.
Khan F, et al.. Molecular targets underlying the anticancer effects of quercetin: an update. Nutrients. 2016;8. https://doi.org/10.3390/nu8090529.
Zhu Y, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-X-L inhibitors, A1331852 and A1155463. Aging. 2017;9:955–63. https://doi.org/10.18632/aging.101202.
Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22(1):78–83. https://doi.org/10.1038/nm.4010.
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15(3):428–35. https://doi.org/10.1111/acel.12445.
Yosef R, Pilpel N, Tokarsky-Amiel R, Biran A, Ovadya Y, Cohen S, et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat Commun. 2016;7:11190. https://doi.org/10.1038/ncomms11190.
Moncsek A, et al. Targeting senescent cholangiocytes and activated fibroblasts with Bcl-xL inhibitors ameliorates fibrosis in Mdr2−/− mice. Hepatology. 2017. https://doi.org/10.1002/hep.29464.
• Fuhrmann-Stroissnigg H, et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun. 2017;8. This study describes a screening platform for identification of novel senotheraputic drugs. https://doi.org/10.1038/s41467-017-00314-z.
Fielding CA, Jones GW, McLoughlin RM, McLeod L, Hammond VJ, Uceda J, et al. Interleukin-6 signaling drives fibrosis in unresolved inflammation. Immunity. 2014;40(1):40–50. https://doi.org/10.1016/j.immuni.2013.10.022.
Chen R, Chen B. Siltuximab (CNTO 328): a promising option for human malignancies. Drug Des Devel Ther. 2015;9:3455–8. https://doi.org/10.2147/DDDT.S86438.
Bose P, Abou Zahr A, Verstovsek S. Investigational Janus kinase inhibitors in development for myelofibrosis. Expert Opin Investig Drugs. 2017;26(6):723–34. https://doi.org/10.1080/13543784.2017.1323871.
Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A. 2015;112(46):E6301–10. https://doi.org/10.1073/pnas.1515386112.
Gadina M, Gazaniga N, Vian L, Furumoto Y. Small molecules to the rescue: Inhibition of cytokine signaling in immune-mediated diseases. J Autoimmun. 2017. https://doi.org/10.1016/j.jaut.2017.06.006.
Wada E, Tanihata J, Iwamura A, Takeda S, Hayashi YK, Matsuda R. Treatment with the anti-IL-6 receptor antibody attenuates muscular dystrophy via promoting skeletal muscle regeneration in dystrophin-/utrophin-deficient mice. Skelet Muscle. 2017;7(1):23. https://doi.org/10.1186/s13395-017-0140-z.
Chakraborty D, Šumová B, Mallano T, Chen CW, Distler A, Bergmann C, et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun. 2017;8(1):1130. https://doi.org/10.1038/s41467-017-01236-6.
Ikeda K, Ueda K, Sano T, Ogawa K, Ikezoe T, Hashimoto Y, et al. The amelioration of myelofibrosis with thrombocytopenia by a JAK1/2 inhibitor, ruxolitinib, in a post-polycythemia vera myelofibrosis patient with a JAK2 exon 12 mutation. Intern Med. 2017;56(13):1705–10. https://doi.org/10.2169/internalmedicine.56.7871.
Komar HM, Serpa G, Kerscher C, Schwoegl E, Mace TA, Jin M, et al. Inhibition of Jak/STAT signaling reduces the activation of pancreatic stellate cells in vitro and limits caerulein-induced chronic pancreatitis in vivo. Sci Rep. 2017;7(1):1787. https://doi.org/10.1038/s41598-017-01973-0.
Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013;15(8):978–90. https://doi.org/10.1038/ncb2784.
Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12(6):325–38. https://doi.org/10.1038/nrneph.2016.48.
Sagiv A, Biran A, Yon M, Simon J, Lowe SW, Krizhanovsky V. Granule exocytosis mediates immune surveillance of senescent cells. Oncogene. 2013;32(15):1971–7. https://doi.org/10.1038/onc.2012.206.
Berger KN, Pu JJ. PD-1 pathway and its clinical application: a 20-year journey after discovery of the complete human PD-1 gene. Gene. 2018;638:20–5. https://doi.org/10.1016/j.gene.2017.09.050.
Acknowledgments
The authors would also like to thank Matthew M. Moore from the Mayo Clinic Center for Innovation for help with the illustration.
Funding
We gratefully acknowledge support from the Glenn/AFAR post-doctoral fellowship program for translational research on aging (M.J.S.), the National Institutes of Health HL092961 (D.J.T.) and AG052958 (N.K.L.), and the John E. and Virginia H. Kunkel Family (N.K.L).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. LeBrasseur has patent killing senescent cells and treating senescence-associated conditions issued and patent compositions and methods for treating senescence-associated diseases and disorders issued. Drs. Schafer, Haak, and Tschumperlin have no conflicts of interest to disclose.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Scleroderma
Rights and permissions
About this article
Cite this article
Schafer, M.J., Haak, A.J., Tschumperlin, D.J. et al. Targeting Senescent Cells in Fibrosis: Pathology, Paradox, and Practical Considerations. Curr Rheumatol Rep 20, 3 (2018). https://doi.org/10.1007/s11926-018-0712-x
Published:
DOI: https://doi.org/10.1007/s11926-018-0712-x