Abstract
Purpose of Review
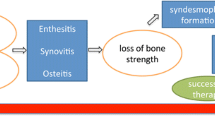
The purpose of the study is to briefly review the molecular mechanisms that leads to structural damage in ankylosing spondylitis (AS), defined as new bone formation resulting in complete or incomplete ankylosis of the spine, and the impact of treatment with biologics to retard this process.
Recent Findings
The understanding of molecular mechanisms leading to new bone formation in AS has significantly improved but is still incomplete. Availability of biologics has greatly enhanced the treatment of patients with AS, but its impact on slowing the structural damage is still a matter of debate, although a few observational studies have shown that long term use of TNF-α blockers may slow radiographic progression. The availability of newer biologics targeting IL-17/1L23 has shown some promising results in slowing radiographic progression in AS.
Summary
Although the availability of TNF-inhibitors has greatly enhanced the treatment options for patients with AS, their impact on slowing the structural damage is still not clearly established. However, preliminary results using newer biologics targeting IL-17/1L23 axis are more encouraging but longer follow-up is needed.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
•• Taurog JD, Chhabra A, Colbert RA. Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med. 2016;374:2563–74. This review article highlights recent advances in our understanding of relationship between spondyloarthritis and ankylosing spondylitis and treatments available.
Khan MA. Ankylosing spondylitis and related spondyloarthropathies: the dramatic advances in the past decade. Rheumatology (Oxford). 2011;50:637–9.
Appel H, Maier R, Loddenkemper C, et al. Immunohistochemical analysis of osteoblasts in zygapophyseal joints of patients with ankylosing spondylitis reveal repair mechanisms similar to osteoarthritis. J Rheumatol. 2010;37:823–8.
Lories RJ, Schett G. Pathophysiology of new bone formation and ankylosis in spondyloarthritis. Rheum Dis Clin N Am. 2012;38:555–67.
Biver E, Hardouin P, Caverzasio J. The “bone morphogenic proteins” pathways in bone and joint diseases: translational perspectives from physiopathology to therapeutic targets. Cytokine Growth Factor Rev. 2013;24:69–81.
Krause C, Guzman A, Knaus P. Noggin. Int J Biochem Cell Biol. 2011;43:478–81.
Lories RJU, Derese I, Luyten FP. Modulation of bone morphogenetic protein signaling inhibits the onset and progression of ankylosing enthesitis. J Clin Invest. 2005;115:1571–9.
Joo YB, Bang SY, Kim TH, Shim SC, Lee S, et al. Bone morphogenetic protein 6 polymorphisms are associated with radiographic progression in ankylosing spondylitis. PLoS One. 2014;9:e104966.
Chen HA, Chen CH, Lin YJ, Chen PC, Chen WS, Lu CL, et al. Association of bone morphogenetic proteins with spinal fusion in ankylosing spondylitis. J Rheumatol. 2010;37:2126–32.
Wendling D, Cedoz JP, Racadot E, Dumoulin G. Serum IL-17, BMP-7, and bone turnover markers in patients with ankylosing spondylitis. Joint Bone Spine. 2007;74:304–5.
El TM, Reis RL. Progenitor and stem cells for bone and cartilage regeneration. J Tissue Eng Regen Med. 2009;3:327–37.
Mohanty ST, Kottam L, Gambardella A, Nicklin MJ, Coulton L, Hughes D, et al. Alterations in the self-renewal and differentiation ability of bone marrow mesenchymal stem cells in a mouse model of rheumatoid arthritis. Arthritis Res Ther. 2010;12:R149.
Sun LY, Zhang HY, Feng XB, Hou YY, Lu LW, Fan LM. Abnormality of bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Lupus. 2007;16:121–8.
•• Xie Z, Wang P, Li Y, Deng W, Zhang X, et al. Imbalance between bone morphogenetic protein 2 and noggin induces abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. Arthritis Rheum. 2016;68:430–40. The study revealed a mechanism of pathologic osteogenesis in AS by demonstrating an imbalance between BMP-2 and Noggin secretion in AS patients that possibly leads to abnormal osteogenic differentiation of AS MSCs.
•• Magrey MN, Haqqi T, Haseeb A. Identification of plasma microRNA expression profile in radiographic axial spondyloarthritis—a pilot study. Clin Rheumatol. 2016;35:1323–7. Brief report about microRNA expression in AS and microRNA 34a is differentially expressed in SpA and has been shown to inhibit bone loss.
Goldring SR, Goldring MB. Eating bone or adding it: the Wnt pathway decides. Nat Med. 2007;13:133–4.
Xie W, Zhou L, Li S, Hui T, Chen D. Wnt/β-catenin signaling plays a key role in the development of spondyloarthritis. Ann N Y Acad Sci. 2016;1364:25–31.
Uderhardt S, Diarra D, Katzenbeisser J, David JP, Zwerina J, et al. Blockade of Dickkopf (DKK)-1 induces fusion of sacroiliac joints. Ann Rheum Dis. 2010;3:592–7.
Kwon SR, Lim MJ, Suh CH, Park SG, Hong YS, Yoon BY, et al. Dickkopf-1 level is lower in patients with ankylosing spondylitis than in healthy people and is not influenced by anti-tumor necrosis factor therapy. Rheumatol Int. 2012;32:2523–7.
Heiland GR, Appel H, Poddubnyy D, Zwerina J, Hueber A, et al. High level of functional dickkopf-1 predicts protection from syndesmophyte formation in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71:572–4.
Appel H, Ruiz-Heiland G, Listing J, Zwerina J, Herrmann M, et al. Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2009;60:3257–62.
González-Chávez SA, Quiñonez-Flores CM, Pacheco-Tena C. Molecular mechanisms of bone formation in spondyloarthritis. Joint Bone Spine. 2016;83:394–400.
Jacques P, Lambrecht S, Verheugen E, Pauwels E, Kollias G, Armaka M, et al. Proof of concept: enthesitis and new bone formation in spondyloarthritis are driven by mechanical strain and stromal cells. Ann Rheum Dis. 2014;73:437–45.
•• Shaw AT, Gravallese EM. Mediators of inflammation and bone remodeling in rheumatic disease. Semin Cell Dev Biol. 2016;49:2–10. This review article discusses the effects of cytokines on bone in two settings, rheumatoid arthritis and spondyloarthritis.
Walsh NC, Reinwald S, Manning CA, et al. Osteoblast function is compromised at sites of focal bone erosion in inflammatory arthritis. J Bone Miner Res. 2009;24:1572–85.
Braun J, Bollow M, Neure L, Seipelt E, Seyrekbasan F, Herbst H, et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 1995;38:499–505.
Im CH, Kang EH, Ki JY, et al. Receptor activator of nuclear factor kappa B ligand-mediated osteoclastogenesis is elevated in ankylosing spondylitis. Clin Exp Rheumatol. 2009;27:620–5.
Sieper J, Poddubnyy D. Inflammation, new bone formation and treatment options in axial spondyloarthritis. Ann Rheum Dis. 2014;73:1439–41.
Pedersen SJ, Sorensen IJ, Lambert RG, Hermann KG, Garnero P, Johansen JS, et al. Radiographic progression is associated with resolution of systemic inflammation in patients with axial spondylarthritis treated with tumor necrosis factor a inhibitors: a study of radiographic progression, inflammation on magnetic resonance imaging, and circulating biomarkers of inflammation, angiogenesis, and cartilage and bone turnover. Arthritis Rheum. 2011;63:3789–800.
Lories RJ, Derese I, de Bari C, Luyten FP. Evidence for uncoupling of inflammation and joint remodeling in a mouse model of spondylarthritis. Arthritis Rheum. 2007;56:489–97.
van der Heijde D, Salonen D, Weissman BN, Landewé R, Maksymowych WP, Kupper H, et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther. 2009;11:R127.
van der Heijde D et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum. 2008;58:3063–70.
van der Heijde D, Landewé R, Einstein S, Ory P, Vosse D, Ni L, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum. 2008;58:1324–31.
Baraliakos X, Haibel H, Listing J, Sieper J, Braun J. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann Rheum Dis. 2014;73:710–5.
•• Haroon N, Inman RD, Learch TJ, et al. The impact of tumor necrosis factor alpha inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2013;65:2645–54. This observational study shows that long term use of TNF inhibitors decreases the odds of radiographic progression in AS.
•• Osta B, Benedetti G, Miossec P. Classical and paradoxical effects of TNF-α on bone homeostasis. Front Immunol. 2014;5:48. A thorough review about the effect on TNF-α on bone homeostasis.
Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T. TNF alpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappa B signaling pathway. Bone. 2009;45:367–76.
Huang H, Zhao N, Xu X, Xu Y, Li S, Zhang J. Dose-specific effects of tumor necrosis factor alpha on osteogenic differentiation of mesenchymal stem cells. Cell Prolif. 2011;44:420–7.
Daoussis D, Liossis SN, Solomou EE, Tsanaktsi A, Bounia K, Karampetsou M, et al. Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum. 2010;62:150–8.
van Duivenvoorde LM, Dorris ML, Satumtira N, van Tok MN, Redlich K, Tak PP, et al. Relationship between inflammation, bone destruction, and osteoproliferation in the HLA-B27/human β2-microglobulin-transgenic rat model of spondylarthritis. Arthritis Rheum. 2012;64:3210–9.
Poddubnyy D, Haibel H, Listing J, Märker-Hermann E, Zeidler H, Braun J, et al. Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum. 2012;64:1388–98.
•• Baraliakos X, Heldmann F, Callhoff J, Listing J, Appelboom T, Brandt J, et al. Which spinal lesions are associated with new bone formation in patients with ankylosing spondylitis treated with anti-TNF agents? A long-term observational study using MRI and conventional radiography. Ann Rheum Dis. 2014;73:1819–25. The study revealed that combination of acute inflammation on STIR sequence (fat-suppressed sequence) and fatty lesions are most predictive of syndesmophyte formation in AS.
Maksymowych WP, Chiowchanwisawakit P, Clare T, et al. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum. 2009;60:93–102.
Rudwaleit M, Jurik AG, Hermann KG, Landewe R, van der Heijde D, Baraliakos X, et al. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis:a consensual approach by the ASAS/OMERACT MRI group. Ann Rheum Dis. 2009;68:1520–7.
•• Bleil J, Maier R, Hempfing A, Sieper J, Appel H, Syrbe U. Granulation tissue eroding the subchondral bone also promotes new bone formation in ankylosing spondylitis. Arthritis Rheum. 2016;68:2456–65. Granulation tissue and not fatty metaplasia may be promoting new bone formation in AS.
Maksymowych WP, Morency N, Conner-Spady B, Lambert RG. Suppression of inflammation and effects on new bone formation in ankylosing spondylitis: evidence for a window of opportunity in disease modification. Ann Rheum Dis. 2013;72:23–8.
•• Ward MM, Deodhar A, Akl EA, Lui A, Ermann J, Gensler LS, et al. American college of rheumatology/spondylitis association of america/spondyloarthritis research and treatment network 2015 recommendations for the treatment of ankylosing spondylitis and nonradiographic axial spondyloarthritis. Arthritis Care Res (Hoboken). 2016;68:151–66. ACR/SPARTAN recommendation for treatment of AS.
Rohekar S, Chan J, Tse SM, Haroon N, Chandran V, Bessette L, et al. 2014 update of the Canadian rheumatology association/spondyloarthritis research consortium of Canada treatment recommendations for the management of spondyloarthritis. Part II: specific management recommendations. J Rheumatol. 2015;42:665–81.
Baraliakos X, van den Berg R, Braun J, van der Heijde D. Update of the literature review on treatment with biologics as a basis for the first update of the ASAS/EULAR management recommendations of ankylosing spondylitis. Rheumatology (Oxford). 2012;51:1378–87.
Sieper J, Poddubnyy D. New evidence on the management of spondyloarthritis. Nat Rev Rheumatol. 2016;12:282–95.
Maas F, Spoorenberg A, Brouwer E, Bos R, Efde M, Chaudhry RN, et al. Spinal radiographic progression in patients with ankylosing spondylitis treated with TNF-α blocking therapy: a prospective longitudinal observational cohort study. PLoS One. 2015;10(4):e0122693.
•• Maas F, Arends S, Brouwer E, Essers I, van der Veer E, Efde M, et al. Reduction in spinal radiographic progression in ankylosing spondylitis patients receiving prolonged treatment with TNF-α inhibitors. Arthritis Care Res (Hoboken). 2016. doi:10.1002/acr.23097. A longitudinal observational cohort study in daily clinical practice showed an overall slow and linear spinal radiographic progression in AS patients with long-term TNF-α blocking therapy.
van der Heijde D, Baraliakos X, Hermann KG, Landewé R, Machado P, Maksymowych W, Davies O, de Peyrecave N, Hoepken B, Bauer L, Nurminen T, Braun J. Four year imaging outcomes in patients with axial spondyloarthritis treated with certolizumab pegol, including patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10).
Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-gammat+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18:1069–76.
Smith JA, Colbert RA. The IL-23/IL-17 axis in spondyloarthritis pathogenesis: Th17 and beyond. Arthritis Rheum. 2014;66:231–41.
Appel H, Maier R, Wu P, Scheer R, Hempfing A, Kayser R, et al. Analysis of IL- 17(+) cells in facet joints of patients with spondyloarthritis suggests that the innate immune pathway might be of greater relevance than the Th17-mediated adaptive immune response. Arthritis Res Ther. 2011;13:R95.
Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009;60:1647–56.
McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–24.
van Tok M, van Duivenvoorde L, et al. Anti-IL17A treatment blocks new bone formation in experimental spondyloarthritis in HLA-B27 transgenic rats. Arthritis Rheumatol. 2015; 67 (suppl 10) [abstract].
•• Braun J, Baraliakos X, Deodhar A, Baeten D, Sieper J, Emery P, Readie A, Martin R, Mpofu S, Richards HB. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann Rheum Dis. 2016 Dec 13. The paper describes the results from the international phase III MEASURE 1 study which showed low mean progression rates in spinal radiographic change with the anti-interleukin 17A antibody, secukinumab in patients with AS.
Boersma JW. Retardation of ossification of the lumbar vertebral column in ankylosing spondylitis by means of phenylbutazone. Scand J Rheumatol. 1976;5:60–4.
Wanders A, Heijde D, Landewe R, et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005;52:1756–65.
Poddubnyy D, Rudwaleit M, Haibel H, et al. Effect of nonsteroidal anti-inflammatory drugs on radiographic spinal progression in patients with axial spondyloarthritis: results from the German Spondyloarthritis Inception Cohort. Ann Rheum Dis. 2012;71:1616–22.
Lories RJ, Haroon N. Bone formation in axial spondyloarthritis. Best Pract Res Clin Rheumatol. 2014;28:765–77.
•• Sieper J, Listing J, Poddubnyy D, et al. Effect of continuous versus on-demand treatment of ankylosing spondylitis with diclofenac over 2 years on radiographic progression of the spine: results from a randomized multicenter trial (ENRADAS). Ann Rheum Dis. 2016;75:1438–43. The study could not confirm the disease modifying effect of NSAIDs in AS.
Acknowledgements
We would like to thank Dr. Irving Kushner, Dr. Stanley Ballou, and Dr. Maria Antonelli for reviewing the paper and making necessary edits.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Magrey reports personal fees from UCB PHARMA and personal fees from Jansenn, outside the submitted work.
Dr. Khan declares no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors
Additional information
This article is part of the Topical Collection on Spondyloarthritis
Rights and permissions
About this article
Cite this article
Magrey, M.N., Khan, M.A. The Paradox of Bone Formation and Bone Loss in Ankylosing Spondylitis: Evolving New Concepts of Bone Formation and Future Trends in Management. Curr Rheumatol Rep 19, 17 (2017). https://doi.org/10.1007/s11926-017-0644-x
Published:
DOI: https://doi.org/10.1007/s11926-017-0644-x