Abstract
Wear debris-induced osteolysis remains the greatest limitation of long-term success for total joint replacements with ultra-high molecular weight polyethylene (UHMWPE) bearings. To address oxidative degradation post-gamma irradiation, manufacturers are investigating the incorporation of antioxidants into PE resins. Similarly, larger molecular weight monomers have been developed to increase crosslinking and decrease wear debris, and ultimately osteolysis. However, the effects of modifying monomer size, crosslink density, and antioxidant incorporation on UHMWPE particle-induced osteoclastic bone resorption and coupled osteoblastic bone formation have never been tested. Here, we review the field of antioxidant-containing UHMWPE, and present an illustrative pilot study evaluating the osteolytic and osteogenic potential of wear debris generated from three chemically distinct particles (MARATHON®, XLK, and AOX™) as determined by a novel 3D micro-CT algorithm designed for the murine calvaria model. The results demonstrate an approach by which the potential osteoprotective effects of antioxidants in UHMWPE can be evaluated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total hip replacement (THR) is most commonly performed for end-stage osteoarthritis, although this procedure is also performed for rarer conditions such as rheumatoid arthritis, avascular necrosis of the hip, and femoral neck fractures. Despite its well-known propensity to generate wear debris and subsequent periprosthetic osteolysis, polyethylene bearing surfaces remain the gold standard for THR based on their consistent results and survivorship of ~85 % after 15 years [1], and the significant pitfalls of alternative articulation designs (i.e. metal-on-metal and ceramic-on-ceramic) [2••, 3]. Thus, a major focus of ultra high molecular weight polyethylene (UHMWPE) research has been on formulations that minimize host response that leads to aseptic loosening.
One of the consequences of early UHMWPE devices, which are sterilized using high-dose gamma irradiation (25−100 kGy), while exposed to air to also crosslink the polymer chains, is the formation of free radicals that become trapped in the final product [4]. These residual reactive oxygen species (ROS), left unaddressed, cause oxidative degradation as seen in UHMWPE components stored on the shelf for a long time in air-permeable packaging prior to implantation [5], leading to increased wear and decreased performance in vivo [6]. Additionally, ROS is known to increase the host response to wear debris [7].
Subsequent improvements in UHMWPE processes introduced temperature-driven manufacturing operations, specifically remelting and annealing, which increases the molecular mobility and facilitates recombination of the free radicals. These additional processes are not without drawbacks, leading either to reduced mechanical properties or only giving a partial protection against oxidation due to ROS [8••]. While annealing methods that heat the UHMWPE to temperatures just below their peak melting point have been adopted to reduce ROS in the manufacturing process, the implants may still be sterilized with gamma irradiation after final packaging, exposing the devices to potentially significant oxidation [9•].
Yet another approach to stabilizing UHMWPE is to provide oxidation resistance without decreasing UHMWPE fatigue strength, by means of the incorporation of antioxidants such as vitamin E into the resin [10], or by diffusing it into already consolidated and radiated UHMWPE [11•]. This advance adds yet another variable to the chemical composition and molecular response to the irradiation/annealing conditions that can be used to generate novel UHMWPE implants. Since the in vivo wear debris properties of each of these constructs will need to be empirically determined to define their effects on osteoclastic bone resorption (osteolysis) and coupled bone formation (osteogenesis), there is a great need for cost-effective in vitro and in vivo models [12, 13], and the pros and cons of the current murine models have recently been reviewed [14].
Our research, aimed at understanding the biological responses to wear debris particles, has relied heavily on the murine calvaria model, which was originally developed to study titanium and polymethylmethacrylate particles with histology [15, 16]. Subsequently, this model has been used as a small animal surrogate to study novel interventions for wear debris-induced osteolysis including: bisphosphonates, cyclo-oxygenase inhibitors, TNF and RANKL biologic antagonists, NF-kappa B inhibitors, Jun kinase inhibitors, inhibitors of the NALP3 inflammasome, and adenosine receptor activators [16–26, 27••]. Most recently, this murine calvaria model was modified to study UHMWPE particles [28], using volumetric micro-CT [18]. Since osteolysis is the result of uncoupled bone resorption, an important component of the murine calvaria model is the robust bone formation that occurs on resorption surfaces within 2 weeks of wear debris implantation [29], which occurs in the complete absence of osteoclasts [22]. This allows for the analysis of both osteolysis and osteogenesis from longitudinal micro-CT data if a faithful 3D registration algorithm can be developed to quantify the wear debris-induced osteolysis (day 0 calvaria bone volume – day 10 calvaria bone volume) and osteogenesis (day 10 osteoid and under-mineralized bone volume). Here, we describe these methods in a pilot study evaluating the effects of particles from three distinct UHMWPE materials (MARATHON, XLK, and AOX), versus sham surgery and hydrogel (PVA-PAA) particle controls (Table 1), aimed at testing the hypothesis that UHMWPE particles of similar size distribution would illicit similar biological response, despite variations in the starting resin or the presence of the antioxidant.
Wear Debris Particle Generation
Particles from three different compositions of UHMWPE (MARATHON, XLK, AOX) were generated from DePuy production barstock lots using high speed cryomilling and cryopulverization (BioEngineering Solutions, Chicago, IL, USA). Particle filtering was used to isolate particles in the 1−10 um range, and they were characterized using low angle laser light scattering (Microtrac-X100,) and scanning electron microscopy with EDS to confirm particle size, shape, and composition. The major differential characteristics of these particles are summarized in Table 2. Particles were EtO sterilized and verified free of endotoxins (<0.01 uE; Kinetic QCL).
In Vivo Studies
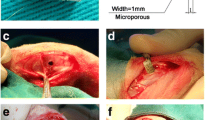
All animal studies were performed under protocols approved by the University of Rochester Committee for Animal Resources. Eight-week-old C57B/6 mice were shaved prior to calvaria surgery, and the area was sterilized with 70 % ethanol and iodine. A 0.5– × 0.5-cm area of calvarial bone was exposed by making a midline sagittal incision over the calvaria, leaving the periosteum intact. A low threshold dose of particles known to be required to induce osteolysis (2 mg) or a high dose (5 mg) of MARATHON, XLK, AOX and hydrogel (PVA-PAA) control were spread over the area of each mouse and hydrogel were directly injected onto the calvaria surface (n = 6). Afterwards, the incision was closed with 2.0 interrupted sutures. One assigned group consisting of an incision of only the skin served as a sham surgery control.
Micro-CT Scanning and Osteolysis vs. Osteogenesis Analysis
Micro-CT scans were performed with a VivaCT40 (ScanCo Medical, Basserdorf, Switzerland) using an isometric resolution of 15 um. Baseline calvaria volume was obtained from in vivo scans on day 0 (before surgery), while the mice were anesthetized with 2 % isofluorine and 1 L/min oxygen. After sacrifice on day 10, the skulls were rescanned with the same parameters. The DICOM micro-CT files were then transferred to Amira v.5.4 (Visage Imaging, San Diego, CA, USA) for quantitative analysis. Quantification of the osteolytic and osteogenic volume was performed as show Fig. 1. Briefly, in vivo micro-CT scans of the calvaria are performed prior to surgical implantation of the wear debris particles (day 0), and ex vivo scans of the calvaria are performed after tissue harvest on day 10. The DICOM files are used to generate an initial 3D image of the calvaria at each time point, and these images are then imported into the Amira program for volumetric registration and analyses. Three distinct tissues types were defined by this process based on their bone mineral density (BMD). In the region of interest (ROI), the original calvarial bone, which has a high BMD (zoom and data window = 1,000–7,000; display and masking = 1,700–7,000), was initially identified. Then an under-mineralized tissue with a lower BMD (zoom and data window = 1,000–3,000; display and masking = 1,000–2,500), was identified within the ROI, which we defined as new bone that formed in response to the wear debris-induced osteolysis. Finally, the unmineralized soft tissue, with a lower BMD, was identified within the ROI between the original calvaria, and the new woven bone was defined as inflammatory tissue. Based on this tissue segmentation, we were able to calculate the volumes and determine the osteolytic versus osteogenic potential of the different particles via liner regression analysis (Fig. 1).
Longitudinal micro-CT analysis and quantification of UHMWPE particle induced osteolysis and osteogenesis in vivo. 3D reconstructions of DICOM images were generated from the micro-CT raw data of the region of interest (ROI) (circled bone) obtained on day 0 and day 10 following surgical implantation of 2 mg of UHMWPE particles. After ROI confirmation via Amira image analysis, the day 0 (yellow bone) and day 10 (green bone) ROIs are co-registered in 3D. The osteolytic volume is then determined by the bone void in the day 10 ROI, and the osteogenic volume is calculated from the under calcified bone in the ROI of the co-registered 3D images. To assess the relative osteolytic versus osteogenic potential of a particular UHMWPE particle, a linear regression analysis is performed by plotting the osteolysis versus the osteogenic volume for each mouse (n = 6), in which slope = 1.0 signifies perfect coupling
The results from the sham and hydrogel-treated calvaria demonstrated minimal osteolysis and the lack of a significant osteogenesis response in these groups. In contrast, all three particles displayed a significant osteolytic and osteogenic effect vs. sham controls (p < 0.01). Additionally, all three particles demonstrated a dose-dependent effect on both osteolysis and osteogenesis, in which the effects of the 5-mg dose appeared to saturate the host responses. Therefore, lower doses are recommended for future studies. The results from the 2-mg dose produced some interesting trends that warrant further investigation. Of note was that the MARATHON particles induced the smallest osteolytic response among the UHMWPE particles tested, suggesting that at low doses it elicits the best biological response. However, when we analyzed the osteolytic versus osteogenic potential of the particles, the MARATHON particles appear to have uncoupled osteolysis and osteogenesis with a slope = 0.59 (Fig. 1). Thus, this uncoupling highlights the importance of measuring both bone responses when evaluating UHMWPE particles for biocompatibility and toxicity. In contrast, AOX particles slightly favor osteogenesis over osteolysis (slope = 1.27). This suggests that the presence of the antioxidant may produce a more favorable environment for bone formation following wear debris-induced bone resorption.
Conclusions and Future Directions
Under non-pathologic conditions, wear debris-induced bone resorption is coupled to bone formation to prevent osteolysis. This is why only a small fraction of joint replacement patients develop periprosthetic osteolysis and aseptic loosening, while most patients display a linear wear rate over time [30–32]. The theory that aseptic loosing may be due to an uncoupling of osteogenic/osteolytic processes rather than a specific negative wear debris responses is supported by longitudinal volumetric CT analysis of patients with varying degrees of periprosthetic bone loss [31–33]. It is for this reason that we have chosen micro-CT as the primary outcome measure in our preclinical studies, and aimed to develop faithful quantitative measures of coupled vs. uncoupled responses to wear debris. Here, we demonstrate that volumetric longitudinal micro-CT can be used to quantify these events using the murine calvaria model. Using this model, we observed differences in the induced osteolysis. Material factors which may have contributed to these differences include the average molecular weight of the resin (5 vs. 2 million), the presence or absence of the antioxidant, but also the average particle size. In this experiment, attempts were made to control for the particle size, but filtering still produces a distribution of particle sizes with significant overlap, which is an issue that requires attention in the future. Moreover, inclusion of anti-oxidants into the larger particles from lower molecular weight resin without remelting results in UHMWPE (AOX) that has similar osteolytic and osteogenic properties to low dose MARATHON, suggesting a biological effect of the anti-oxidants that compensates for the lack of ROS release from remelting.
One limitation of our pilot study is that the UHMWPE particles differed in more than one variable (Tables 1 and 2). Thus, we are not able to make firm conclusions about the effects of anti-oxidant incorporation. However, it was interesting to see that the AOX particles induced less osteolysis than the XLK particles, and significantly more osteogenesis than the MARATHON (p < 0.05). Formal studies are now planned to directly assess the potential effect of a free radical scavenging antioxidant presence on the overall osteolysis process.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gomez-Barrena E, Medel F, Puertolas JA. Polyethylene oxidation in total hip arthroplasty: evolution and new advances. Open Orthop J. 2009;3:115–20.
•• Graves SE, et al. A multinational assessment of metal-on-metal bearings in hip replacement. J Bone Joint Surg Am. 2012;93 Suppl 3:43–7. Highly cited reference describing early failure problems with metal-on-metal implants.
Garcia-Cimbrelo E, et al. Mittelmeier ceramic-ceramic prosthesis after 10 years. J Arthroplasty. 1996;11:773–81.
Jahan M, et al. A study of long-lived free radicals in gamma-irradiated medical grade polyethlene. Radiat Phys Chem. 2001;62:141–4.
Currier BH, et al. Shelf life and in vivo duration. Impacts on performance of tibial bearings. Clin Orthop Relat Res. 1997;342:111–22.
Gomez-Barrena E, et al. Role of polyethylene oxidation and consolidation defects in cup performance. Clin Orthop Relat Res. 1998;352:105–17.
Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bull NYU Hosp Jt Dis. 2009;67:182–8.
•• Atwood SA, et al. Tradeoffs amongst fatigue, wear, and oxidation resistance of cross-linked ultra-high molecular weight polyethylene. J Mech Behav Biomed Mater. 2011;4:1033–45. This is the first study to simultaneously evaluate fatigue crack propagation, wear, oxidation, and microstructure in a wide variety of clinically-relevant ultra-high molecular weight polyethylene. The trade-off we have shown in fatigue, wear, and oxidation performance is critical to the material's long-term success in total joint replacements.
• Kurtz SM, et al. Mechanical properties, oxidation, and clinical performance of retrieved highly cross-linked Crossfire liners after intermediate-term implantation. J Arthroplasty. 2009;25:614–23. e611-612. Highly cited reference on the properties and clinical performance of UHMWPE bearings.
Lidgren L, et al. The invention relates to a method for the preparation in a reactor of a UHMWPE material doped with an antioxidant, preferably vitamin E. US patent# 6448315. 2002.
• Oral E, Muratoglu OK. Vitamin E diffused, highly crosslinked UHMWPE: a review. Int Orthop. 2010;35:215–23. Highly cited reference on the incorporation of vitamin E into UHMWPE bearings.
Ingham E, Fisher J. Biological reactions to wear debris in total joint replacement. Proc Inst Mech Eng H. 2000;214:21–37.
Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–86.
Purdue PE, et al. The central role of wear debris in periprosthetic osteolysis. HSS J. 2006;2:102–13.
Merkel KD, et al. Tumor necrosis factor-alpha mediates orthopedic implant osteolysis. Am J Pathol. 1999;154:203–10.
Schwarz EM, et al. Quantitative small-animal surrogate to evaluate drug efficacy in preventing wear debris-induced osteolysis. J Orthop Res. 2000;18:849–55.
von Knoch M, et al. The decrease of particle-induced osteolysis after a single dose of bisphosphonate. Biomaterials. 2005;26:1803–8.
Tsutsumi R, et al. Differential effects of biologic versus bisphosphonate inhibition of wear debris-induced osteolysis assessed by longitudinal micro-CT. J Orthop Res. 2008;26:1340–6.
Goater JJ, et al. Efficacy of ex vivo OPG gene therapy in preventing wear debris induced osteolysis. J Orthop Res. 2002;20:169–73.
Zhang X, et al. Evidence for a direct role of cyclo-oxygenase 2 in implant wear debris-induced osteolysis. J Bone Miner Res. 2001;16:660–70.
Childs LM, et al. Efficacy of etanercept for wear debris-induced osteolysis. J Bone Miner Res. 2001;16:338–47.
Childs LM, et al. In vivo RANK signaling blockade using the receptor activator of NF-kappaB:Fc effectively prevents and ameliorates wear debris-induced osteolysis via osteoclast depletion without inhibiting osteogenesis. J Bone Miner Res. 2002;17:192–9.
Clohisy JC, et al. NF-kB signaling blockade abolishes implant particle-induced osteoclastogenesis. J Orthop Res. 2004;22:13–20.
Clohisy JC, et al. Inhibition of IKK activation, through sequestering NEMO, blocks PMMA-induced osteoclastogenesis and calvarial inflammatory osteolysis. J Orthop Res. 2006;24:1358–65.
Yamanaka Y, et al. Map kinase c-JUN N-terminal kinase mediates PMMA induction of osteoclasts. J Orthop Res. 2006;24:1349–57.
Burton L, et al. Orthopedic wear debris mediated inflammatory osteolysis is mediated in part by NALP3 inflammasome activation. J Orthop Res. 2012;31:73–80.
•• Mediero A, et al. Adenosine A2A receptor activation prevents wear particle-induced osteolysis. Sci Transl Med. 2012;4:135ra165. Novel therapeutic approach to prevent wear debris-induced osteolysis.
Taki N, et al. Polyethylene and titanium particles induce osteolysis by similar, lymphocyte-independent, mechanisms. J Orthop Res. 2005;23:376–83.
Kaar SG, et al. Rapid repair of titanium particle-induced osteolysis is dramatically reduced in aged mice. J Orthop Res. 2001;19:171–8.
Martell JM, Berdia S. Determination of polyethylene wear in total hip replacements with use of digital radiographs. J Bone Joint Surg Am. 1997;79:1635–41.
Looney RJ, et al. Volumetric computerized tomography as a measurement of periprosthetic acetabular osteolysis and its correlation with wear. Arthritis Res. 2002;4:59–63.
Howie DW, et al. Progression of acetabular periprosthetic osteolytic lesions measured with computed tomography. J Bone Joint Surg Am. 2007;89:1818–25.
Schwarz EM, et al. Use of volumetric computerized tomography as a primary outcome measure to evaluate drug efficacy in the prevention of peri-prosthetic osteolysis: a 1-year clinical pilot of etanercept vs. placebo. J Orthop Res. 2003;21:1049–55.
Acknowledgments
The authors would like to thank Ryan Tierney and Michael Thullen for technical assistance with the histology and micro-CT analyses, respectively. We also thank Drs. Matthew Dressler and Todd Render for their helpful input. This work was supported by the Orthopaedic Research and Education Foundation; the National Institutes of Health PHS awards P50 AR054041 and P30 AR061307; and DePuy J&J Inc.
Conflict of Interest
Justin M. Green has received research grant support from DePuy J&J.
Nadim J. Hallab has received support for particle production from DePuy J&J and has served as a consultant for DePuy J&J, Smith and Nephew, Medtronic, Orthopedic Analysis LLC, and BioEngineering Solutions.
Yen-Shuo Liao has been employed by DePuy Orthopaedics.
Venkat Narayan has been employed by DePuy Orthopaedics.
Edward M. Schwarz has received research grant support from, served as a consultant for, and received lecturing honoraria from DePuy J&J, and is the founder and president of LAGeT, Inc.
Chao Xie has received research grant support from DePuy J&J.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Osteoarthritis
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Green, J.M., Hallab, N.J., Liao, YS. et al. Anti-oxidation Treatment of Ultra High Molecular Weight Polyethylene Components to Decrease Periprosthetic Osteolysis: Evaluation of Osteolytic and Osteogenic Properties of Wear Debris Particles in a Murine Calvaria Model. Curr Rheumatol Rep 15, 325 (2013). https://doi.org/10.1007/s11926-013-0325-3
Published:
DOI: https://doi.org/10.1007/s11926-013-0325-3