Abstract
Purpose of Review
Osteoblasts are responsible for bone matrix production during bone development and homeostasis. Much is known about the transcriptional regulation and signaling pathways governing osteoblast differentiation. However, less is known about how osteoblasts obtain or utilize nutrients to fulfill the energetic demands associated with osteoblast differentiation and bone matrix synthesis. The goal of this review is to highlight and discuss what is known about the role and regulation of bioenergetic metabolism in osteoblasts with a focus on more recent studies.
Recent Findings
Bioenergetic metabolism has emerged as an important regulatory node in osteoblasts. Recent studies have begun to identify the major nutrients and bioenergetic pathways favored by osteoblasts as well as their regulation during differentiation. Here, we highlight how osteoblasts obtain and metabolize glucose, amino acids, and fatty acids to provide energy and other metabolic intermediates. In addition, we highlight the signals that regulate nutrient uptake and metabolism and focus on how energetic metabolism promotes osteoblast differentiation.
Summary
Bioenergetic metabolism provides energy and other metabolites that are critical for osteoblast differentiation and activity. This knowledge contributes to a more comprehensive understanding of osteoblast biology and may inform novel strategies to modulate osteoblast differentiation and bone anabolism in patients with bone disorders.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Yang X, Matsuda K, Bialek P, Jacquot S, Masuoka HC, Schinke T, et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology; implication for Coffin-Lowry syndrome. Cell. 2004;117(3):387–98. https://doi.org/10.1016/s0092-8674(04)00344-7.
Elefteriou F, Benson MD, Sowa H, Starbuck M, Liu X, Ron D, et al. ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae. Cell Metab. 2006;4(6):441–51. https://doi.org/10.1016/j.cmet.2006.10.010.
Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nature Reviews Cancer. 2016;16(10):619–34. https://doi.org/10.1038/nrc.2016.71.
• Lee W-C, Ji X, Nissim I, Long F. Malic enzyme couples mitochondria with aerobic glycolysis in osteoblasts. Cell Reports. 2020;32(10):108108. https://doi.org/10.1016/j.celrep.2020.108108This study demonstrates that increased expression of malic enzyme ME2 promotes glycolytic flux into malate aspartate shuttle during osteoblast differentiation. Malate aspartate shuttle replenishes NAD+ to further facilitate glycolysis.
Carey BW, Finley LW, Cross JR, Allis CD, Thompson CB. Intracellular α-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518(7539):413–6. https://doi.org/10.1038/nature13981.
Stegen S, Laperre K, Eelen G, Rinaldi G, Fraisl P, Torrekens S, et al. HIF-1α metabolically controls collagen synthesis and modification in chondrocytes. Nature. 2019;565(7740):511–5. https://doi.org/10.1038/s41586-019-0874-3.
D'Aniello C, Cermola F, Palamidessi A, Wanderlingh LG, Gagliardi M, Migliaccio A, et al. Collagen Prolyl Hydroxylation–Dependent Metabolic Perturbation Governs Epigenetic Remodeling and Mesenchymal Transition in Pluripotent and Cancer Cells. Cancer Research. 2019;79(13):3235–50. https://doi.org/10.1158/0008-5472.Can-18-2070.
Birsoy K, Wang T, Chen WW, Freinkman E, Abu-Remaileh M, Sabatini DM. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell. 2015;162(3):540–51. https://doi.org/10.1016/j.cell.2015.07.016.
Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander Heiden MG. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell. 2015;162(3):552–63. https://doi.org/10.1016/j.cell.2015.07.017.
• Gao J, Feng Z, Wang X, Zeng M, Liu J, Han S, et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018;25(2):229–40. https://doi.org/10.1038/cdd.2017.144This study shows that mitochondrial activity increases along with elevated oxidative stress during osteoblast differentiation. In response, SIRT3 enhances SOD2 activity to mitigate the oxidative stress to support osteoblast differentiation.
Nagano T, Nakashima A, Onishi K, Kawai K, Awai Y, Kinugasa M, et al. Proline dehydrogenase promotes senescence through the generation of reactive oxygen species. J Cell Sci. 2017;130(8):1413–20. https://doi.org/10.1242/jcs.196469.
Komarova SV, Ataullakhanov FI, Globus RK. Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am J Physiol Cell Physiol. 2000;279(4):C1220–9. https://doi.org/10.1152/ajpcell.2000.279.4.C1220.
• Guntur AR, Gerencser AA, Le PT, DeMambro VE, Bornstein SA, Mookerjee SA, et al. Osteoblast-like MC3T3-E1 cells prefer glycolysis for ATP production but adipocyte-like 3T3-L1 cells prefer oxidative phosphorylation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2018;33(6):1052–65. https://doi.org/10.1002/jbmr.3390This study shows that differentiated osteoblasts preferentially rely on glycolysis for ATP production, while differentiated adipocytes mainly use oxidative phosphorylation.
• Müller DIH, Stoll C, Palumbo-Zerr K, Böhm C, Krishnacoumar B, Ipseiz N, et al. PPARδ-mediated mitochondrial rewiring of osteoblasts determines bone mass. Scientific Reports. 2020;10(1):8428. https://doi.org/10.1038/s41598-020-65305-5This study shows that PPARδ is required for the increased oxidative phosphorylation during osteoblast differentiation. Deletion of PPARδ impairs osteoblast differentiation and reduces bone mass.
• Dobson PF, Dennis EP, Hipps D, Reeve A, Laude A, Bradshaw C, et al. Mitochondrial dysfunction impairs osteogenesis, increases osteoclast activity, and accelerates age related bone loss. Scientific reports. 2020;10(1):11643. https://doi.org/10.1038/s41598-020-68566-2This article demonstrates that mitochondrial dysfunction caused by mutations in mitochondrial DNA polymerase results in bone loss due to reduced osteoblast differentiation and increased osteoclast activity.
Wojtovich AP, Smith CO, Haynes CM, Nehrke KW, Brookes PS. Physiological consequences of complex II inhibition for aging, disease, and the mKATP channel. Biochim Biophys Acta. 2013;1827(5):598–611. https://doi.org/10.1016/j.bbabio.2012.12.007.
Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324(5930):1076–80. https://doi.org/10.1126/science.1164097.
Karner CM, Esen E, Chen J, Hsu FF, Turk J, Long F. Wnt protein signaling reduces nuclear Acetyl-CoA levels to suppress gene expression during osteoblast differentiation. J Biol Chem. 2016;291(25):13028–39. https://doi.org/10.1074/jbc.M115.708578.
An JH, Yang JY, Ahn BY, Cho SW, Jung JY, Cho HY, et al. Enhanced mitochondrial biogenesis contributes to Wnt induced osteoblastic differentiation of C3H10T1/2 cells. Bone. 2010;47(1):140–50. https://doi.org/10.1016/j.bone.2010.04.593.
• Shares BH, Busch M, White N, Shum L, Eliseev RA. Active mitochondria support osteogenic differentiation by stimulating β-catenin acetylation. J Biol Chem. 2018;293(41):16019–27. https://doi.org/10.1074/jbc.RA118.004102This study shows that stimulating OXPHOS stabilizes β-catenin through acetylation and in turn promotes osteoblast differentiation. This paper highlights metabolic alterations can directly interact with osteogenic signaling pathways.
Shi X, Zhang Y, Zheng J, Pan J. Reactive oxygen species in cancer stem cells. Antioxid Redox Signal. 2012;16(11):1215–28. https://doi.org/10.1089/ars.2012.4529.
Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011;7(8):504–11. https://doi.org/10.1038/nchembio.607.
Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–62. https://doi.org/10.1016/j.cub.2014.03.034.
Galloway CA, Yoon Y. Perspectives on: SGP symposium on mitochondrial physiology and medicine: what comes first, misshape or dysfunction? The view from metabolic excess. J Gen Physiol. 2012;139(6):455–63. https://doi.org/10.1085/jgp.201210771.
Guntur AR, Le PT, Farber CR, Rosen CJ. Bioenergetics during calvarial osteoblast differentiation reflect strain differences in bone mass. Endocrinology. 2014;155(5):1589–95. https://doi.org/10.1210/en.2013-1974.
Kobayashi K, Nojiri H, Saita Y, Morikawa D, Ozawa Y, Watanabe K, et al. Mitochondrial superoxide in osteocytes perturbs canalicular networks in the setting of age-related osteoporosis. Scientific Reports. 2015;5(1):9148. https://doi.org/10.1038/srep09148.
Shum LC, Hollenberg AM, Baldwin AL, Kalicharan BH, Maqsoodi N, Rubery PT, et al. Role of oxidative metabolism in osseointegration during spinal fusion. PLOS ONE. 2020;15(11):e0241998. https://doi.org/10.1371/journal.pone.0241998.
• Shares BH, Smith CO, Sheu TJ, Sautchuk R Jr, Schilling K, Shum LC, et al. Inhibition of the mitochondrial permeability transition improves bone fracture repair. Bone. 2020;137:115391. https://doi.org/10.1016/j.bone.2020.115391This study demonstrates that protecting mitochondrial integrity through inhibition of the opening of mitochondrial permeability transition pores promotes fracture healing.
Finkel T. From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci Signal. 2012;5(215):pe10. https://doi.org/10.1126/scisignal.2002943.
Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45(5):549–61. https://doi.org/10.1016/j.freeradbiomed.2008.05.004.
Mochin MT, Underwood KF, Cooper B, McLenithan JC, Pierce AD, Nalvarte C, et al. Hyperglycemia and redox status regulate RUNX2 DNA-binding and an angiogenic phenotype in endothelial cells. Microvasc Res. 2015;97:55–64. https://doi.org/10.1016/j.mvr.2014.09.008.
Arai M, Shibata Y, Pugdee K, Abiko Y, Ogata Y. Effects of reactive oxygen species (ROS) on antioxidant system and osteoblastic differentiation in MC3T3-E1 cells. IUBMB Life. 2007;59(1):27–33. https://doi.org/10.1080/15216540601156188.
Hinoi E, Fujimori S, Wang L, Hojo H, Uno K, Yoneda Y. Nrf2 negatively regulates osteoblast differentiation via interfering with Runx2-dependent transcriptional activation. J Biol Chem. 2006;281(26):18015–24. https://doi.org/10.1074/jbc.M600603200.
Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, Abraham E. Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem. 2010;285(43):33154–64. https://doi.org/10.1074/jbc.M110.143685.
Hinchy EC, Gruszczyk AV, Willows R, Navaratnam N, Hall AR, Bates G, et al. Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. Journal of Biological Chemistry. 2018;293(44):17208–17. https://doi.org/10.1074/jbc.RA118.002579.
Wei J, Shimazu J, Makinistoglu MP, Maurizi A, Kajimura D, Zong H, et al. Glucose uptake and Runx2 synergize to orchestrate osteoblast differentiation and bone formation. Cell. 2015;161(7):1576–91. https://doi.org/10.1016/j.cell.2015.05.029.
Peck WA, Birge SJ, Fedak SA. Bone cells: biochemical and biological studies after enzymatic isolation. Science. 1964;146(3650):1476–7. https://doi.org/10.1126/science.146.3650.1476.
Zoch ML, Abou DS, Clemens TL, Thorek DL, Riddle RC. In vivo radiometric analysis of glucose uptake and distribution in mouse bone. Bone Res. 2016;4:16004. https://doi.org/10.1038/boneres.2016.4.
Esen E, Lee SY, Wice BM, Long F. PTH promotes bone anabolism by stimulating aerobic glycolysis via IGF signaling. J Bone Miner Res. 2015;30(11):1959–68. https://doi.org/10.1002/jbmr.2556.
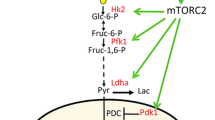
Esen E, Chen J, Karner CM, Okunade AL, Patterson BW, Long F. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab. 2013;17(5):745–55. https://doi.org/10.1016/j.cmet.2013.03.017.
Zoidis E, Ghirlanda-Keller C, Schmid C. Stimulation of glucose transport in osteoblastic cells by parathyroid hormone and insulin-like growth factor I. Mol Cell Biochem. 2011;348(1-2):33–42. https://doi.org/10.1007/s11010-010-0634-z.
Li Z, Frey JL, Wong GW, Faugere MC, Wolfgang MJ, Kim JK, et al. Glucose transporter-4 facilitates insulin-stimulated glucose uptake in osteoblasts. Endocrinology. 2016;157(11):4094–103. https://doi.org/10.1210/en.2016-1583.
• Chen H, Ji X, Lee WC, Shi Y, Li B, Abel ED, et al. Increased glycolysis mediates Wnt7b-induced bone formation. Faseb J. 2019;33(7):7810–21. https://doi.org/10.1096/fj.201900201RRThis study shows that WNT-7b stimulates glucose consumption and glycolysis, which in turn increases bone mass. Deletion of GLUT1 reduces WNT-7b induced high bone mass phenotype.
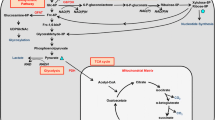
Karner CM, Long F. Glucose metabolism in bone. Bone. 2018;115:2–7. https://doi.org/10.1016/j.bone.2017.08.008.
Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510(7504):298–302. https://doi.org/10.1038/nature13236.
Yang M, Vousden KH. Serine and one-carbon metabolism in cancer. Nature Reviews Cancer. 2016;16(10):650–62. https://doi.org/10.1038/nrc.2016.81.
Warburg O. Uber den stoffwechsel der karzinomezellen. Biochem Z. 1924;152:309–44.
• Lee SY, Abel ED, Long F. Glucose metabolism induced by Bmp signaling is essential for murine skeletal development. Nat Commun. 2018;9(1):4831. https://doi.org/10.1038/s41467-018-07316-5This paper shows that GLUT1 is required for chondrocyte proliferation, matrix production and maturation during endochondral ossification. BMP signaling regulates GLUT1 expression via mTORC1-Hif1a cascade.
• Misra BB, Jayapalan S, Richards AK, Helderman RCM, Rendina-Ruedy E. Untargeted metabolomics in primary murine bone marrow stromal cells reveals distinct profile throughout osteoblast differentiation. Metabolomics. 2021;17(10):86. https://doi.org/10.1007/s11306-021-01829-9This study provides a complete metabolomic data set of bone marrow stromal cells during osteoblast differentiation.
Wu Y, Wang M, Feng H, Peng Y, Sun J, Qu X, et al. Lactate induces osteoblast differentiation by stabilization of HIF1α. Mol Cell Endocrinol. 2017;452:84–92. https://doi.org/10.1016/j.mce.2017.05.017.
Peek CB, Levine DC, Cedernaes J, Taguchi A, Kobayashi Y, Tsai SJ, et al. Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab. 2017;25(1):86–92. https://doi.org/10.1016/j.cmet.2016.09.010.
Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325–37. https://doi.org/10.1038/nrc3038.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (New York, NY). 2009;324(5930):1029–33. https://doi.org/10.1126/science.1160809.
Dirckx N, Moorer MC, Clemens TL, Riddle RC. The role of osteoblasts in energy homeostasis. Nature Reviews Endocrinology. 2019;15(11):651–65. https://doi.org/10.1038/s41574-019-0246-y.
Regan JN, Lim J, Shi Y, Joeng KS, Arbeit JM, Shohet RV, et al. Up-regulation of glycolytic metabolism is required for HIF1α-driven bone formation. Proc Natl Acad Sci U S A. 2014;111(23):8673–8. https://doi.org/10.1073/pnas.1324290111.
• Hong M, Zhang XB, Xiang F, Fei X, Ouyang XL, Peng XC. MiR-34a suppresses osteoblast differentiation through glycolysis inhibition by targeting lactate dehydrogenase-A (LDHA). In Vitro Cell Dev Biol Anim. 2020;56(6):480–7. https://doi.org/10.1007/s11626-020-00467-0This study demonstrates that miR-34a inhibits the expression of glycolytic genes and suppresses osteoblast differentiation in part by directly targeting LDHA.
Zhang D, Bae C, Lee J, Lee J, Jin Z, Kang M, et al. The bone anabolic effects of irisin are through preferential stimulation of aerobic glycolysis. Bone. 2018;114:150–60. https://doi.org/10.1016/j.bone.2018.05.013.
• Jin Z, Kho J, Dawson B, Jiang M-M, Chen Y, Ali S, et al. Nitric oxide modulates bone anabolism through regulation of osteoblast glycolysis and differentiation. The Journal of Clinical Investigation. 2021;131(5):e138935. https://doi.org/10.1172/JCI138935This study shows that arginine metabolism provides nitric oxide that activates glycolysis to support osteoblast differentiation and bone formation. This study highlights the interaction between amino acid metabolism and glycolysis.
• Lee SY, Long F. Notch signaling suppresses glucose metabolism in mesenchymal progenitors to restrict osteoblast differentiation. J Clin Invest. 2018;128(12):5573–86. https://doi.org/10.1172/jci96221This study reveals that Notch signaling suppresses glycolysis via reduced phosphorylation of AMPK. Inhibition of glycolysis reverses the high bone mass phenotype of Notch2 mutant mice.
• Hollenberg AM, Smith CO, Shum LC, Awad H, Eliseev RA. Lactate dehydrogenase inhibition with oxamate exerts bone anabolic effect. Journal of Bone and Mineral Research. 2020;35(12):2432–43. https://doi.org/10.1002/jbmr.4142This study found that inhibition of lactate dehydrogenase using oxamate stimulates OXPHOS and promotes osteoblast differentiation and increases bone mass.
Thornburg JM, Nelson KK, Clem BF, Lane AN, Arumugam S, Simmons A, et al. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res. 2008;10(5):R84. https://doi.org/10.1186/bcr2154.
Phang JM, Liu W, Hancock CN, Fischer JW. Proline metabolism and cancer: emerging links to glutamine and collagen. Curr Opin Clin Nutr Metab Care. 2015;18(1):71–7. https://doi.org/10.1097/MCO.0000000000000121.
Hosios AM, Hecht VC, Danai LV, Johnson MO, Rathmell JC, Steinhauser ML, et al. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev Cell. 2016;36(5):540–9. https://doi.org/10.1016/j.devcel.2016.02.012.
Krall AS, Xu S, Graeber TG, Braas D, Christofk HR. Asparagine promotes cancer cell proliferation through use as an amino acid exchange factor. Nat Commun. 2016;7:11457. https://doi.org/10.1038/ncomms11457.
• Yu Y, Newman H, Shen L, Sharma D, Hu G, Mirando AJ, et al. Glutamine metabolism regulates proliferation and lineage allocation in skeletal stem cells. Cell Metab. 2019;29(4):966–78 e4. https://doi.org/10.1016/j.cmet.2019.01.016This paper demonstrates glutaminase-mediated glutamine metabolism is required for skeletal stem cell proliferation and both osteoblast specification and differentiation. Mechanistically, transaminase dependent α-ketoglutarate production is required for SSC proliferation.
• Stegen S, Devignes CS, Torrekens S, Van Looveren R, Carmeliet P, Carmeliet G. Glutamine Metabolism in osteoprogenitors is required for bone mass accrual and PTH-induced bone anabolism in male Mice. J Bone Miner Res. 2020. https://doi.org/10.1002/jbmr.4219This paper shows that PTH promotes glutamine consumption and catabolism in osteoblasts. Mechanistically, glutamine metabolism contributes to amino acid and nucleotide biosynthesis required for osteoblast differentiation, and glutathione production to promote osteoblast viability.
• Shen L, Sharma D, Yu Y, Long F, Karner CM. Biphasic regulation of glutamine consumption by WNT during osteoblast differentiation. Journal of Cell Science. 2021;134(1):jcs251645. https://doi.org/10.1242/jcs.251645This study shows that WNT regulates glutamine consumption through two amino acid transporters via distinct signaling pathways. WNT rapidly induces the expression Slc7a7 via β-catenin signaling pathway, while Slc1a5 is regulated via mTORC1-ATF4 cascade for sustained glutamine consumption.
• Sharma D, Yu Y, Shen L, Zhang G-F, Karner CM. SLC1A5 provides glutamine and asparagine necessary for bone development in mice. eLife. 2021;10:e71595. https://doi.org/10.7554/eLife.71595This study found the neutral amino acid transporter SLC1A5 provides glutamine and asparagine to regulate protein synthesis and osteoblast differentiation. Mechanistically, glutamine and asparagine are used to synthesize non-essential amino acids to support osteoblast differentiation.
Hu G, Yu Y, Tang YJ, Wu C, Long F, Karner CM. The amino acid sensor Eif2ak4/GCN2 is required for proliferation of osteoblast progenitors in mice. Journal of Bone and Mineral Research. 2020;35(10):2004–14. https://doi.org/10.1002/jbmr.4091.
Karner CM, Esen E, Okunade AL, Patterson BW, Long F. Increased glutamine catabolism mediates bone anabolism in response to WNT signaling. J Clin Invest. 2015;125(2):551–62. https://doi.org/10.1172/JCI78470.
Wang Y, Deng P, Liu Y, Wu Y, Chen Y, Guo Y, et al. Alpha-ketoglutarate ameliorates age-related osteoporosis via regulating histone methylations. Nature Communications. 2020;11(1):5596. https://doi.org/10.1038/s41467-020-19360-1.
• Stegen S, Rinaldi G, Loopmans S, Stockmans I, Moermans K, Thienpont B, et al. Glutamine metabolism controls chondrocyte identity and function. Dev Cell. 2020;53(5):530–44.e8. https://doi.org/10.1016/j.devcel.2020.05.001This study highlights the multi-functional role of glutamine in chondrocytes. Glutamine metabolism contributes to the epigenetic regulation of chondrogenic genes, aspartate synthesis for cell proliferation and matrix synthesis, and glutathione synthesis to offset ROS for cell survival.
Tencerova M, Figeac F, Ditzel N, Taipaleenmaki H, Nielsen TK, Kassem M. High-fat diet-induced obesity promotes expansion of bone marrow adipose tissue and impairs skeletal stem cell functions in mice. J Bone Miner Res. 2018;33(6):1154–65. https://doi.org/10.1002/jbmr.3408.
Stegen S, van Gastel N, Eelen G, Ghesquiere B, D'Anna F, Thienpont B, et al. HIF-1alpha promotes glutamine-mediated redox homeostasis and glycogen-dependent bioenergetics to support postimplantation bone cell survival. Cell Metab. 2016;23(2):265–79. https://doi.org/10.1016/j.cmet.2016.01.002.
Adamek G, Felix R, Guenther HL, Fleisch H. Fatty acid oxidation in bone tissue and bone cells in culture. Characterization and hormonal influences. The Biochemical journal. 1987;248(1):129–37. https://doi.org/10.1042/bj2480129.
Niemeier A, Niedzielska D, Secer R, Schilling A, Merkel M, Enrich C, et al. Uptake of postprandial lipoproteins into bone in vivo: impact on osteoblast function. Bone. 2008;43(2):230–7. https://doi.org/10.1016/j.bone.2008.03.022.
• Kim SP, Li Z, Zoch ML, Frey JL, Bowman CE, Kushwaha P, et al. Fatty acid oxidation by the osteoblast is required for normal bone acquisition in a sex- and diet-dependent manner. JCI Insight. 2017;2(16):e92704. https://doi.org/10.1172/jci.insight.92704This study demonstrates that fatty acid oxidation mediated by CPT2 is critical for bone formation in female mice.
Rendina-Ruedy E, Guntur AR, Rosen CJ. Intracellular lipid droplets support osteoblast function. Adipocyte. 2017;6(3):250–8. https://doi.org/10.1080/21623945.2017.1356505.
Frey JL, Li Z, Ellis JM, Zhang Q, Farber CR, Aja S, et al. Wnt-Lrp5 signaling regulates fatty acid metabolism in the osteoblast. Mol Cell Biol. 2015;35(11):1979–91. https://doi.org/10.1128/mcb.01343-14.
• van Gastel N, Stegen S, Eelen G, Schoors S, Carlier A, Daniëls VW, et al. Lipid availability determines fate of skeletal progenitor cells via SOX9. Nature. 2020;579(7797):111–7. https://doi.org/10.1038/s41586-020-2050-1This study reveals that lipid scarcity induces chondrogenesis of skeletal progenitors through activation of SOX9 expression via FOXO during bone healing. In turn, SOX9 further suppresses fatty acid oxidation, allowing cells to adapt to the avascular environment with nutrient restriction.
Wang Y, Li Y, Mao K, Li J, Cui Q, Wang GJ. Alcohol-induced adipogenesis in bone and marrow: a possible mechanism for osteonecrosis. Clin Orthop Relat Res. 2003;410:213–24. https://doi.org/10.1097/01.blo.0000063602.67412.83.
McGee-Lawrence ME, Carpio LR, Schulze RJ, Pierce JL, McNiven MA, Farr JN, et al. Hdac3 deficiency Increases marrow adiposity and induces lipid storage and glucocorticoid metabolism in osteochondroprogenitor cells. Journal of Bone and Mineral Research. 2016;31(1):116–28. https://doi.org/10.1002/jbmr.2602.
Maurel DB, Boisseau N, Benhamou CL, Jaffre C. Alcohol and bone: review of dose effects and mechanisms. Osteoporos Int. 2012;23(1):1–16. https://doi.org/10.1007/s00198-011-1787-7.
Enlow DH, Conklin JL, Bang S. Observations on the occurrence and the distribution of lipids in compact bone. Clin Orthop Relat Res. 1965;38:157–69. https://doi.org/10.1097/00003086-196500380-00022.
Bensaad K, Favaro E, Lewis CA, Peck B, Lord S, Collins JM, et al. Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 2014;9(1):349–65. https://doi.org/10.1016/j.celrep.2014.08.056.
• Maridas DE, Rendina-Ruedy E, Helderman RC, DeMambro VE, Brooks D, Guntur AR, et al. Progenitor recruitment and adipogenic lipolysis contribute to the anabolic actions of parathyroid hormone on the skeleton. Faseb j. 2019;33(2):2885–98. https://doi.org/10.1096/fj.201800948RRThis study shows that PTH promotes lipolysis in adipocytes to release fatty acids. Fatty acids are taken up by neighboring osteoblasts and promote their differentiation.
Krall AS, Mullen PJ, Surjono F, Momcilovic M, Schmid EW, Halbrook CJ, et al. Asparagine couples mitochondrial respiration to ATF4 activity and tumor growth. Cell Metabolism. 2021;33(5):1013–26.e6. https://doi.org/10.1016/j.cmet.2021.02.001.
Wu Q, ba-alawi W, Deblois G, Cruickshank J, Duan S, Lima-Fernandes E, et al. GLUT1 inhibition blocks growth of RB1-positive triple negative breast cancer. Nature Communications. 2020;11(1):4205. https://doi.org/10.1038/s41467-020-18020-8.
Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 2014;13(4):890–901. https://doi.org/10.1158/1535-7163.Mct-13-0870.
Elgogary A, Xu Q, Poore B, Alt J, Zimmermann SC, Zhao L, et al. Combination therapy with BPTES nanoparticles and metformin targets the metabolic heterogeneity of pancreatic cancer. Proceedings of the National Academy of Sciences. 2016;113(36):E5328–E36. https://doi.org/10.1073/pnas.1611406113.
Fairfield H, Falank C, Harris E, Demambro V, McDonald M, Pettitt JA, et al. The skeletal cell-derived molecule sclerostin drives bone marrow adipogenesis. J Cell Physiol. 2018;233(2):1156–67. https://doi.org/10.1002/jcp.25976.
Li W, Deng Y, Feng B, Mak KK. Mst1/2 Kinases modulate glucose uptake for osteoblast differentiation and bone formation. J Bone Miner Res. 2018;33(6):1183–95. https://doi.org/10.1002/jbmr.3413.
• Dirckx N, Tower RJ, Mercken EM, Vangoitsenhoven R, Moreau-Triby C, Breugelmans T, et al. Vhl deletion in osteoblasts boosts cellular glycolysis and improves global glucose metabolism. J Clin Invest. 2018;128(3):1087–105. https://doi.org/10.1172/JCI97794This study demonstrates that stabilization of HIF1a promotes bone formation via increased glycolysis. The study also highlights the association between glucose metabolism in osteoblasts and whole body glucose homeostasis.
Yao Q, Khan MP, Merceron C, LaGory EL, Tata Z, Mangiavini L, et al. Suppressing mitochondrial respiration is critical for hypoxia tolerance in the fetal growth plate. Dev Cell. 2019;49(5):748–63.e7. https://doi.org/10.1016/j.devcel.2019.04.029.
Ambrogini E, Almeida M, Martin-Millan M, Paik JH, Depinho RA, Han L, et al. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 2010;11(2):136–46. https://doi.org/10.1016/j.cmet.2009.12.009.
Rached MT, Kode A, Xu L, Yoshikawa Y, Paik JH, Depinho RA, et al. FoxO1 is a positive regulator of bone formation by favoring protein synthesis and resistance to oxidative stress in osteoblasts. Cell Metab. 2010;11(2):147–60. https://doi.org/10.1016/j.cmet.2010.01.001.
Kim J-H, Singhal V, Biswal S, Thimmulappa RK, DiGirolamo DJ. Nrf2 is required for normal postnatal bone acquisition in mice. Bone Research. 2014;2(1):14033. https://doi.org/10.1038/boneres.2014.33.
Funding
Work in the Karner lab is supported by National Institute of Health R01 grants (AR076325 and AR071967) to C.M.K.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Skeletal Biology and Regulation
Rights and permissions
About this article
Cite this article
Shen, L., Hu, G. & Karner, C.M. Bioenergetic Metabolism In Osteoblast Differentiation. Curr Osteoporos Rep 20, 53–64 (2022). https://doi.org/10.1007/s11914-022-00721-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-022-00721-2