Abstract
Purpose of Review
We will review non-renal-related mechanisms of fibroblast growth factor 23 (FGF23) pathophysiology.
Recent Findings
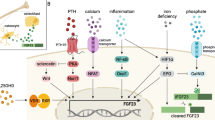
FGF23 production and metabolism may be affected by many bone, mineral, and kidney factors. However, it has recently been demonstrated that other factors, such as iron status, erythropoietin, and inflammation, also affect FGF23 production and metabolism. As these non-mineral factors are especially relevant in the setting of chronic kidney disease (CKD), they may represent emerging determinants of CKD-associated elevated FGF23 levels. Moreover, FGF23 itself may promote anemia and inflammation, thus contributing to the multifactorial etiologies of these CKD-associated comorbidities.
Summary
CKD-relevant, non-mineral-related, bidirectional relationships exist between FGF23 and anemia, and between FGF23 and inflammation. Iron deficiency, anemia, and inflammation affect FGF23 production and metabolism, and FGF23 itself may contribute to anemia and inflammation, highlighting complex interactions that may affect aspects of CKD pathogenesis and treatment.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004;113(4):561–8. https://doi.org/10.1172/jci19081.
Gattineni J, Bates C, Twombley K, Dwarakanath V, Robinson ML, Goetz R, et al. FGF23 decreases renal NaPi-2a and NaPi-2c expression and induces hypophosphatemia in vivo predominantly via FGF receptor 1. Am J Physiol Ren Physiol. 2009;297(2):F282–91. https://doi.org/10.1152/ajprenal.90742.2008.
Bai XY, Miao D, Goltzman D, Karaplis AC. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem. 2003;278(11):9843–9. https://doi.org/10.1074/jbc.M210490200.
Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429–35. https://doi.org/10.1359/jbmr.0301264.
Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96(1):365–408. https://doi.org/10.1152/physrev.00014.2015.
Pereira RC, Juppner H, Azucena-Serrano CE, Yadin O, Salusky IB, Wesseling-Perry K. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone. 2009;45(6):1161–8. https://doi.org/10.1016/j.bone.2009.08.008.
Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–8. https://doi.org/10.1038/ki.2011.47.
Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64(6):2272–9. https://doi.org/10.1046/j.1523-1755.2003.00328.x.
Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205–15. https://doi.org/10.1681/asn.2005010052.
Portale AA, Wolf M, Juppner H, Messinger S, Kumar J, Wesseling-Perry K, et al. Disordered FGF23 and mineral metabolism in children with CKD. Clin J Am Soc Nephrol. 2014;9(2):344–53. https://doi.org/10.2215/cjn.05840513.
Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–408. https://doi.org/10.1172/jci46122.
Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119(19):2545–52. https://doi.org/10.1161/circulationaha.108.844506.
Mitsnefes MM, Betoko A, Schneider MF, Salusky IB, Wolf MS, Juppner H, et al. FGF23 and left ventricular hypertrophy in children with CKD. Clin J Am Soc Nephrol. 2018;13(1):45–52. https://doi.org/10.2215/cjn.02110217.
Dai B, David V, Martin A, Huang J, Li H, Jiao Y, et al. A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PLoS One. 2012;7(9):e44161. https://doi.org/10.1371/journal.pone.0044161.
Smith ER, Holt SG, Hewitson TD. FGF23 activates injury-primed renal fibroblasts via FGFR4-dependent signalling and enhancement of TGF-beta autoinduction. Int J Biochem Cell Biol. 2017;92:63–78. https://doi.org/10.1016/j.biocel.2017.09.009.
Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, et al. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18(9):2600–8. https://doi.org/10.1681/asn.2006080936.
Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–9. https://doi.org/10.1001/jama.2011.826.
Portale AA, Wolf MS, Messinger S, Perwad F, Juppner H, Warady BA, et al. Fibroblast growth factor 23 and risk of CKD progression in children. Clin J Am Soc Nephrol. 2016;11(11):1989–98. https://doi.org/10.2215/cjn.02110216.
Rossaint J, Oehmichen J, Van Aken H, Reuter S, Pavenstadt HJ, Meersch M, et al. FGF23 signaling impairs neutrophil recruitment and host defense during CKD. J Clin Invest. 2016;126(3):962–74. https://doi.org/10.1172/jci83470.
Chonchol M, Greene T, Zhang Y, Hoofnagle AN, Cheung AK. Low vitamin D and high fibroblast growth factor 23 serum levels associate with infectious and cardiac deaths in the HEMO study. J Am Soc Nephrol. 2016;27(1):227–37. https://doi.org/10.1681/asn.2014101009.
Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–92. https://doi.org/10.1056/NEJMoa0706130.
Komaba H, Fukagawa M. The role of FGF23 in CKD--with or without Klotho. Nat Rev Nephrol. 2012;8(8):484–90. https://doi.org/10.1038/nrneph.2012.116.
Tagliabracci VS, Engel JL, Wiley SE, Xiao J, Gonzalez DJ, Nidumanda Appaiah H, et al. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci U S A. 2014;111(15):5520–5. https://doi.org/10.1073/pnas.1402218111.
Yamamoto H, Ramos-Molina B, Lick AN, Prideaux M, Albornoz V, Bonewald L, et al. Posttranslational processing of FGF23 in osteocytes during the osteoblast to osteocyte transition. Bone. 2016;84:120–30. https://doi.org/10.1016/j.bone.2015.12.055.
Wolf M, White KE. Coupling fibroblast growth factor 23 production and cleavage: iron deficiency, rickets, and kidney disease. Curr Opin Nephrol Hypertens. 2014;23(4):411–9. https://doi.org/10.1097/01.mnh.0000447020.74593.6f.
Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, et al. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;95(2):578–85. https://doi.org/10.1210/jc.2009-1603.
Smith ER, Cai MM, McMahon LP, Holt SG. Biological variability of plasma intact and C-terminal FGF23 measurements. J Clin Endocrinol Metab. 2012;97(9):3357–65. https://doi.org/10.1210/jc.2012-1811.
Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, et al. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280(4):2543–9. https://doi.org/10.1074/jbc.M408903200.
Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91(8):3144–9. https://doi.org/10.1210/jc.2006-0021.
Wesseling-Perry K, Pereira RC, Sahney S, Gales B, Wang HJ, Elashoff R, et al. Calcitriol and doxercalciferol are equivalent in controlling bone turnover, suppressing parathyroid hormone, and increasing fibroblast growth factor-23 in secondary hyperparathyroidism. Kidney Int. 2011;79(1):112–9. https://doi.org/10.1038/ki.2010.352.
Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Ren Physiol. 2010;299(4):F882–9. https://doi.org/10.1152/ajprenal.00360.2010.
Lopez I, Rodriguez-Ortiz ME, Almaden Y, Guerrero F, de Oca AM, Pineda C, et al. Direct and indirect effects of parathyroid hormone on circulating levels of fibroblast growth factor 23 in vivo. Kidney Int. 2011;80(5):475–82. https://doi.org/10.1038/ki.2011.107.
Shimada T, Yamazaki Y, Takahashi M, Hasegawa H, Urakawa I, Oshima T, et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am J Physiol Ren Physiol. 2005;289(5):F1088–95. https://doi.org/10.1152/ajprenal.00474.2004.
Hu MC, Kuro-o M, Moe OW. Klotho and chronic kidney disease. Contrib Nephrol. 2013;180:47–63. https://doi.org/10.1159/000346778.
Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci U S A. 2011;108(46):E1146–55. https://doi.org/10.1073/pnas.1110905108.
Clinkenbeard EL, Farrow EG, Summers LJ, Cass TA, Roberts JL, Bayt CA, et al. Neonatal iron deficiency causes abnormal phosphate metabolism by elevating FGF23 in normal and ADHR mice. J Bone Miner Res. 2014;29(2):361–9. https://doi.org/10.1002/jbmr.2049.
• David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016;89(1):135–46. https://doi.org/10.1038/ki.2015.290 This study demonstrates the effects of iron deficiency and inflammation on FGF23 production and metabolism.
Hanudel MR, Chua K, Rappaport M, Gabayan V, Valore E, Goltzman D, et al. Effects of dietary iron intake and chronic kidney disease on fibroblast growth factor 23 metabolism in wild-type and hepcidin knockout mice. Am J Physiol Ren Physiol. 2016;311(6):F1369–f77. https://doi.org/10.1152/ajprenal.00281.2016.
Durham BH, Joseph F, Bailey LM, Fraser WD. The association of circulating ferritin with serum concentrations of fibroblast growth factor-23 measured by three commercial assays. Ann Clin Biochem. 2007;44(Pt 5):463–6. https://doi.org/10.1258/000456307781646102.
Imel EA, Peacock M, Gray AK, Padgett LR, Hui SL, Econs MJ. Iron modifies plasma FGF23 differently in autosomal dominant hypophosphatemic rickets and healthy humans. J Clin Endocrinol Metab. 2011;96(11):3541–9. https://doi.org/10.1210/jc.2011-1239.
Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28(8):1793–803. https://doi.org/10.1002/jbmr.1923.
Ali FN, Josefson J, Mendez AJ, Mestan K, Wolf M. Cord blood ferritin and fibroblast growth Factor-23 levels in neonates. J Clin Endocrinol Metab. 2016;101(4):1673–9. https://doi.org/10.1210/jc.2015-3709.
Imel EA, Liu Z, McQueen AK, Acton D, Acton A, Padgett LR, et al. Serum fibroblast growth factor 23, serum iron and bone mineral density in premenopausal women. Bone. 2016;86:98–105. https://doi.org/10.1016/j.bone.2016.03.005.
Lewerin C, Ljunggren O, Nilsson-Ehle H, Karlsson MK, Herlitz H, Lorentzon M, et al. Low serum iron is associated with high serum intact FGF23 in elderly men: the Swedish MrOS study. Bone. 2017;98:1–8. https://doi.org/10.1016/j.bone.2017.02.005.
Zhang Q, Doucet M, Tomlinson RE, Han X, Quarles LD, Collins MT, et al. The hypoxia-inducible factor-1alpha activates ectopic production of fibroblast growth factor 23 in tumor-induced osteomalacia. Bone Res. 2016;4:16011. https://doi.org/10.1038/boneres.2016.11.
Hanudel MR, Wesseling-Perry K, Gales B, Ramos G, Campbell V, Ethridge K, et al. Effects of acute kidney injury and chronic hypoxemia on fibroblast growth factor 23 levels in pediatric cardiac surgery patients. Pediatr Nephrol. 2016;31(4):661–9. https://doi.org/10.1007/s00467-015-3257-5.
McMahon S, Grondin F, McDonald PP, Richard DE, Dubois CM. Hypoxia-enhanced expression of the proprotein convertase furin is mediated by hypoxia-inducible factor-1: impact on the bioactivation of proproteins. J Biol Chem. 2005;280(8):6561–9. https://doi.org/10.1074/jbc.M413248200.
Silvestri L, Pagani A, Camaschella C. Furin-mediated release of soluble hemojuvelin: a new link between hypoxia and iron homeostasis. Blood. 2008;111(2):924–31. https://doi.org/10.1182/blood-2007-07-100677.
Munoz Mendoza J, Isakova T, Ricardo AC, Xie H, Navaneethan SD, Anderson AH, et al. Fibroblast growth factor 23 and inflammation in CKD. Clin J Am Soc Nephrol. 2012;7(7):1155–62. https://doi.org/10.2215/cjn.13281211.
Hanks LJ, Casazza K, Judd SE, Jenny NS, Gutierrez OM. Associations of fibroblast growth factor-23 with markers of inflammation, insulin resistance and obesity in adults. PLoS One. 2015;10(3):e0122885. https://doi.org/10.1371/journal.pone.0122885.
Ito N, Wijenayaka AR, Prideaux M, Kogawa M, Ormsby RT, Evdokiou A, et al. Regulation of FGF23 expression in IDG-SW3 osteocytes and human bone by pro-inflammatory stimuli. Mol Cell Endocrinol. 2015;399:208–18. https://doi.org/10.1016/j.mce.2014.10.007.
Durlacher-Betzer K, Hassan A, Levi R, Axelrod J, Silver J, Naveh-Many T. Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 2018;94:315–25. https://doi.org/10.1016/j.kint.2018.02.026.
Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102(3):783–8. https://doi.org/10.1182/blood-2003-03-0672.
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–3. https://doi.org/10.1126/science.1104742.
Clinkenbeard EL, Hanudel MR, Stayrook KR, Appaiah HN, Farrow EG, Cass TA, et al. Erythropoietin stimulates murine and human fibroblast growth factor-23, revealing novel roles for bone and bone marrow. Haematologica. 2017;102(11):e427–e30. https://doi.org/10.3324/haematol.2017.167882.
Rabadi S, Udo I, Leaf DE, Waikar SS, Christov M. Acute blood loss stimulates fibroblast growth factor 23 production. Am J Physiol Ren Physiol. 2018;314(1):F132–f9. https://doi.org/10.1152/ajprenal.00081.2017.
Flamme I, Ellinghaus P, Urrego D, Kruger T. FGF23 expression in rodents is directly induced via erythropoietin after inhibition of hypoxia inducible factor proline hydroxylase. PLoS One. 2017;12(10):e0186979. https://doi.org/10.1371/journal.pone.0186979.
Toro L, Barrientos V, Leon P, Rojas M, Gonzalez M, Gonzalez-Ibanez A, et al. Erythropoietin induces bone marrow and plasma fibroblast growth factor 23 during acute kidney injury. Kidney Int. 2018;93(5):1131–41. https://doi.org/10.1016/j.kint.2017.11.018.
Daryadel A, Bettoni C, Haider T, Imenez Silva PH, Schnitzbauer U, Pastor-Arroyo EM, et al. Erythropoietin stimulates fibroblast growth factor 23 (FGF23) in mice and men. Pflugers Arch. 2018;470:1569–82. https://doi.org/10.1007/s00424-018-2171-7.
Hanudel MR, Eisenga MF, Rappaport M, Chua K, Qiao B, Jung G, et al. Effects of erythropoietin on fibroblast growth factor 23 in mice and humans. Nephrol Dial Transplant. 2018. https://doi.org/10.1093/ndt/gfy189.
Artunc F, Risler T. Serum erythropoietin concentrations and responses to anaemia in patients with or without chronic kidney disease. Nephrol Dial Transplant. 2007;22(10):2900–8. https://doi.org/10.1093/ndt/gfm316.
• Coe LM, Madathil SV, Casu C, Lanske B, Rivella S, Sitara D. FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis. J Biol Chem. 2014;289(14):9795–810. https://doi.org/10.1074/jbc.M113.527150 This study demonstrates how FGF23 may inhibit aspects of erythropoiesis.
Agoro R, Montagna A, Goetz R, Aligbe O, Singh G, Coe LM, et al. Inhibition of fibroblast growth factor 23 (FGF23) signaling rescues renal anemia. FASEB J. 2018. https://doi.org/10.1096/fj.201700667R.
Mehta R, Cai X, Hodakowski A, Lee J, Leonard M, Ricardo A, et al. Fibroblast growth factor 23 and anemia in the chronic renal insufficiency cohort study. Clin J Am Soc Nephrol. 2017;12(11):1795–803. https://doi.org/10.2215/cjn.03950417.
Singh S, Grabner A, Yanucil C, Schramm K, Czaya B, Krick S, et al. Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int. 2016;90(5):985–96. https://doi.org/10.1016/j.kint.2016.05.019.
Acknowledgements
The work in this manuscript has been performed with the support of the National Institute of Diabetes, Digestive, and Kidney Disease of the National Institute of Health research grants K08-DK111980 (MRH) and T32-DK104687 (ML).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mark Hanudel and Marciana Laster declare no conflict of interest.
Isidro Salusky reports grants from Amgen, honoraria and speaker fees from Ultragenyx, and expenses covered for Keryx.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Kidney and Bone
Rights and permissions
About this article
Cite this article
Hanudel, M.R., Laster, M. & Salusky, I.B. Non-renal-Related Mechanisms of FGF23 Pathophysiology. Curr Osteoporos Rep 16, 724–729 (2018). https://doi.org/10.1007/s11914-018-0492-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-018-0492-2