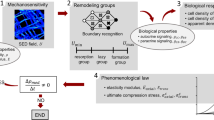
Abstract
Purpose of Review
Connecting organ-scale loads to cellular signals in their local in vivo environment is a current challenge in the field of bone (re)modelling. Understanding this critical missing link would greatly improve our ability to anticipate mechanotransduction during different modes of stimuli and the resultant cellular responses. This review characterises computational approaches that could enable coupling links across the multiple scales of bone.
Recent Findings
Current approaches using strain and fluid shear stress concepts have begun to link organ-scale loads to cellular signals; however, these approaches fail to capture localised micro-structural heterogeneities. Furthermore, models that incorporate downstream communication from osteocytes to osteoclasts, bone-lining cells and osteoblasts, will help improve the understanding of (re)modelling activities. Incorporating this potentially key information in the local in vivo environment will aid in developing multiscale models of mechanotransduction that can predict or help describe resultant biological events related to bone (re)modelling.
Summary
Progress towards multiscale determination of the cell mechanical environment from organ-scale loads remains elusive. Construction of organ-, tissue- and cell-scale computational models that include localised environmental variation, strain amplification and intercellular communication mechanisms will ultimately help couple the hierarchal levels of bone.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Yuan Y, Chen X, Zhang L, Wu J, Guo J, Zou D, et al. The roles of exercise in bone remodeling and in prevention and treatment of osteoporosis. Prog Biophys Mol Biol. 2016;122(2):122–30.
Roux W. Der Kampf der Theile im Oranismus: Ein Beitrag zur verollständigung der mechanischen Zweckmässigkeitslehre. Leipzig: W. Engelmann; 1881.
Wolff J. Das Gesetz der Transformation der Knochen. Hirchwald. 1893 Nov;19(47):1222–4.
Christen P, Müller R. In vivo visualisation and quantification of bone resorption and bone formation from time-lapse imaging. Curr Osteoporos Rep. 2017;15(4):311–7.
•• Birkhold AI, Razi H, Weinkamer R, Duda GN, Checa S, Willie BM. Monitoring in vivo (re)modeling: a computational approach using 4D microCT data to quantify bone surface movements. Bone. 2015;75:210–21. This paper covers the key tools for providing temporal and spatial information essential for development of geometries for computational models at the organ to tissue scale and longitudinal analysis for validation of remodelling simulations.
•• Kameo Y, Adachi T. Interstitial fluid flow in canaliculi as a mechanical stimulus for cancellous bone remodeling: in silico validation. Biomech Model Mechanobiol. 2014;13(4):851–60. This paper provides a good example of an implementation of a poroelastic micro-FE simulation used to model the remodelling process coupled with an in silico validation.
Vaughan TJ, Verbruggen SW, McNamara LM. Are all osteocytes equal? Multiscale modelling of cortical bone to characterise the mechanical stimulation of osteocytes. Int J Numer Method Biomed Eng. 2013;29(12):1361–72.
Schulte FA, Zwahlen A, Lambers FM, Kuhn G, Ruffoni D, Betts D, et al. Strain-adaptive in silico modeling of bone adaptation—a computer simulation validated by in vivo micro-computed tomography data. Bone. 2013;52(1):485–92.
Willie BM, Birkhold AI, Razi H, Thiele T, Aido M, Kruck B, et al. Diminished response to in vivo mechanical loading in trabecular and not cortical bone in adulthood of female C57Bl/6 mice coincides with a reduction in deformation to load. Bone. 2013;55(2):335–46.
Schulte FA, Ruffoni D, Lambers FM, Christen D, Webster DJ, Kuhn G, et al. Local mechanical stimuli regulate bone formation and resorption in mice at the tissue level. PLoS One. 2013;8(4):e62172.
Scheiner S, Pivonka P, Hellmich C. Coupling systems biology with multiscale mechanics, for computer simulations of bone remodeling. Comput Methods Appl Mech Eng. 2013;254:181–96.
Knothe Tate ML, Gunning PW, Sansalone V. Emergence of form from function – mechanical engineering approaches to probe the role of stem cell mechanoadaptation in sealing cell fate. BioArchitecture. 2016 Sep 2;6(5):85–103.
Wang JW, Lu DY, Mao DB, Long M. Mechanomics: an emerging field between biology and biomechanics. Protein Cell. 2014;5(7):518–31.
Trussel A, Müller R, Webster D. Toward mechanical systems biology in bone. Ann Biomed Eng. 2012;40(11):2475–87.
Scheuren A, Wehrle E, Flohr F, Müller R. Bone mechanobiology in mice: toward single-cell in vivo mechanomics. Biomech Model Mechanobiol. 2017 Dec 1;16(6).
Nichterwitz S, Chen G, Benitez JA, Yilmaz M, Storvall H, Cao M, et al. Laser capture microscopy coupled with smart-seq2 for precise spatial transcriptomic profiling. Nat Commun. 2016 Jul 8;7:12139.
Trüssel AJ. Spatial mapping and high throughput microfluidic gene expression analysis of osteocytes in mechanically controlled bone remodeling. 2015 (Doctoral dissertation, ETH Zurich).
Rho JY, Kuhn-Spearing L, Zioupos P. Mechanical properties and the hierarchical structure of bone. Med Eng Phys. 1998;20(2):92–102.
Dermience M, Lognay G, Mathieu F, Goyens P. Effects of thirty elements on bone metabolism. J Trace Elem Med Biol. 2015;32:86–106.
Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38.
Vaughan T, Haugh M, McNamara L. A fluid-structure interaction model to characterize bone cell stimulation in parallel-plate flow chamber systems. J R Soc Interface. 2013;10(81):20120900.
Lambers FM, Kuhn G, Weigt C, Koch KM, Schulte FA, Müller R. Bone adaptation to cyclic loading in murine caudal vertebrae is maintained with age and directly correlated to the local micromechanical environment. J Biomech. 2015;48(6):1179–87.
Metzger TA, Schwaner SA, LaNeve AJ, Kreipke TC, Niebur GL. Pressure and shear stress in trabecular bone marrow during whole bone loading. J Biomech. 2015;48(12):3035–43.
• Lerebours C, Buenzli PR, Scheiner S, Pivonka P. A multiscale mechanobiological model of bone remodelling predicts site-specific bone loss in the femur during osteoporosis and mechanical disuse. Biomech Model Mechanobiol. 2016;15(1):43–67. This paper provides an alternative approach to the standard finite element approach for calculating the mechanical environment, using analytical or semi-analytical methods to model the local mechanical environment.
•• Verbruggen SW, Vaughan TJ, McNamara LM. Fluid flow in the osteocyte mechanical environment: a fluid-structure interaction approach. Biomech Model Mechanobiol. 2014;13(1):85–97. This paper provides an excellent example of coupling fluid and structural mechanics within the cell scale, indicating the importance of a combined structural/fluid appraoch within bone tissue.
Jahani M, Genever PG, Patton RJ, Ahwal F, Fagan MJ. The effect of osteocyte apoptosis on signalling in the osteocyte and bone lining cell network: a computer simulation. J Biomech. 2012;45(16):2876–83.
Verbruggen SW, Vaughan TJ, McNamara LM. Strain amplification in bone mechanobiology: a computational investigation of the in vivo mechanics of osteocytes. J R Soc Interface. 2012;9(75):2735–44.
Mader KS, Schneider P, Müller R, Stampanoni M. A quantitative framework for the 3D characterization of the osteocyte lacunar system. Bone. 2013;57(1):142–54.
Cresswell EN, Nguyen TM, Horsfield MW, Alepuz AJ, Metzger TA, Niebur GL, et al. Mechanically induced bone formation is not sensitive to local osteocyte density in rat vertebral cancellous bone. J Orthop Res. 2018 Feb;36(2):672–81.
Buenzli PR, Sims NA. Quantifying the osteocyte network in the human skeleton. Bone. 2015;75:144–50.
Webster DJ, Morley PL, van Lenthe GH, Müller R. A novel in vivo mouse model for mechanically stimulated bone adaptation—a combined experimental and computational validation study. Comput Methods Biomech Biomed Engin. 2008;11(5):435–41.
Lambers FM, Koch K, Kuhn G, Ruffoni D, Weigt C, Schulte FA, et al. Trabecular bone adapts to long-term cyclic loading by increasing stiffness and normalization of dynamic morphometric rates. Bone. 2013;55(2):325–34.
Lambers FM, Stuker F, Weigt C, Kuhn G, Koch K, Schulte FA, et al. Longitudinal in vivo imaging of bone formation and resorption using fluorescence molecular tomography. Bone. 2013;52(2):587–95.
Cresswell E, Goff M, Nguyen T, Lee W, Hernandez C. Spatial relationships between bone formation and mechanical stress within cancellous bone. Elsevier; 2016 Jan 25;49(2):222–8.
Vanrietbergen B, Weinans H, Huiskes R, Odgaard A. A new method to determine trabecular bone elastic properties and loading using micromechanical finite-element models. J Biomech. 1995;28(1):69–81.
Huiskes R. If bone is the answer, then what is the question? J Anat. 2000;197:145–56.
Kameo Y, Adachi T. Modeling trabecular bone adaptation to local bending load regulated by mechanosensing osteocytes. Acta Mech. 2014;225(10):2833–40.
Pereira AF, Javaheri B, Pitsillides AA, Shefelbine SJ. Predicting cortical bone adaptation to axial loading in the mouse tibia. J R Soc Interface. 2015 Sep 6;12(110):20150590.
Tiwari AK, Prasad J. Computer modelling of bone’s adaptation: the role of normal strain, shear strain and fluid flow. Biomech Model Mechanobiol. 2017;16(2):395–410.
Webster D, Schulte FA, Lambers FM, Kuhn G, Wuller R. Strain energy density gradients in bone marrow predict osteoblast and osteoclast activity: a finite element study. J Biomech. 2015;48(5):866–74.
Metzger TA, Niebur GL. Comparison of solid and fluid constitutive models of bone marrow during trabecular bone compression. J Biomech. 2016;49(14):3596–601.
• Metzger TA, Kreipke TC, Vaughan TJ, McNamara LM, Niebur GL. The in situ mechanics of trabecular bone marrow: the potential for mechanobiological response. J Biomech Eng. 2015 Jan 1;137(1):011006. This paper provides thorough investigation into the mechanics of bone marrow, providing an approach for the investigation into the mechano-response of cells on the surface of the bone tissue and within the marrow.
Scheiner S, Pivonka P, Hellmich C. Poromechanical stimulation of bone remodeling: a continuum micromechanics-based mathematical model and experimental validation. Fifth Biot Conference on Poromechanics 2013. p. 1867–76.
Hellmich C, Kober C, Erdmann B. Micromechanics-based conversion of CT data into anisotropic elasticity tensors, applied to FE simulations of a mandible. Ann Biomed Eng. 2008;36(1):108–22.
You LD, Weinbaum S, Cowin SC, Schaffler MB. Ultrastructure of the osteocyte process and its pericellular matrix. Anat Rec A Discov Mol Cell Evol Biol. 2004;278a(2):505–13.
Anderson EJ, Tate MLK. Idealization of pericellular fluid space geometry and dimension results in a profound underprediction of nano-microscale stresses imparted by fluid drag on osteocytes. J Biomech. 2008;41(8):1736–46.
Bonivtch AR, Bonewald LF, Nicolella DP. Tissue strain amplification at the osteocyte lacuna: a microstructural finite element analysis. J Biomech. 2007;40(10):2199–206.
Wang LP, Dong JH, Xian CJ. Strain amplification analysis of an osteocyte under static and cyclic loading: a finite element study. Biomed Res Int. 2015;2015.
Kamioka H, Kameo Y, Imai Y, Bakker AD, Bacabac RG, Yamada N, et al. Microscale fluid flow analysis in a human osteocyte canaliculus using a realistic high-resolution image-based three-dimensional model. Integr Biol. 2012;4(10):1198–206.
Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010 Dec 1;2(12):a005066.
Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011 Mar 1;3(3):a004994.
Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20(17):4639–47.
Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10(1):21–33.
Luo TZ, Mohan K, Iglesias PA, Robinson DN. Molecular mechanisms of cellular mechanosensing. Nat Mater. 2013;12(11):1063–70.
Nguyen AM, Jacobs CR. Emerging role of primary cilia as mechanosensors in osteocytes. Bone. 2013;54(2):196–204.
Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, Corey DP, et al. Primary cilia are not calcium-responsive mechanosensors. Nature. 2016;531(7596):656–60.
Walker LM, Publicover SJ, Preston MR, Ahmed MAAS, El Haj AJ. Calcium-channel activation and matrix protein upregulation in bone cells in response to mechanical strain. J Cell Biochem. 2000;79(4):648–61.
Dror RO, Dirks RM, Grossman JP, Xu HF, Shaw DE. Biomolecular simulation: a computational microscope for molecular biology. Annu Rev Biophys. 2012;41:429–52.
Jing D, Baik AD, Lu XL, Zhou B, Lai XH, Wang LY, et al. In situ intracellular calcium oscillations in osteocytes in intact mouse long bones under dynamic mechanical loading. FASEB J. 2014;28(4):1582–92.
Lewis KJ, Frikha-Benayed D, Louie J, Stephen S, Spray DC, Thi MM, et al. Osteocyte calcium signals encode strain magnitude and loading frequency in vivo. Proc Natl Acad Sci U S A. 2017;114(44):11775–80.
Ridha H, Almitani KH, Chamekh A, Toumi H, Tavares JMRS. A theory for bone resorption based on the local rupture of osteocytes cells connections: a finite element study. Math Biosci. 2015;262:46–55.
Frost HM. Bone mass and the mechanostat—a proposal. Anat Rec. 1987;219(1):1–9.
Pivonka P, Buenzli PR, Scheiner S, Hellmich C, Dunstan CR. The influence of bone surface availability in bone remodelling—a mathematical model including coupled geometrical and biomechanical regulations of bone cells. Eng Struct. 2013;47:134–47.
Grimal Q, Raum K, Gerisch A, Laugier P. A determination of the minimumsizes of representative volume elements for the prediction of cortical bone elastic properties. Biomech Model Mechanobiol. 2011;10(6):925–37.
Erdemira A, Guess TM, Halloran J, Tadepalli SC, Morrison TM. Considerations for reporting finite element analysis studies in biomechanics. J Biomech. 2012;45(4):625–33.
Jasiuk I. Micromechanics of bone modeled as a composite material. In: Meguid SAaW, George J, (Eds.), Micromechanics and nanomechanics of composite solids. Springer, Cham. 2018. p. 281–306.
Nicolella DP, Moravits DE, Gale AM, Bonewald LF, Lankford J. Osteocyte lacunae tissue strain in cortical bone. J Biomech. 2006;39(9):1735–43.
Begonia M, Dallas M, Johnson ML, Thiagarajan G. Comparison of strain measurement in the mouse forearm using subject-specific finite element models, strain gaging. Biomech Model Mechanobiol. 2017;16(4):1243–53.
Begonia MT, Dallas M, Vizcarra B, Liu Y, Johnson ML, Thiagarajan G. Non-contact strain measurement in the mouse forearm loading model using digital image correlation (DIC). Bone. 2015;81:593–601.
Weinans H, Huiskes R, Grootenboer HJ. The behavior of adaptive bone-remodeling simulation models. J Biomech. 1992;25(12):1425–41.
• Levchuk A, Zwahlen A, Weigt C, Lambers FM, Badilatti SD, Schulte FA, et al. The clinical biomechanics award 2012—presented by the European Society of Biomechanics: large scale simulations of trabecular bone adaptation to loading and treatment. Clin Biomech. 2014;29(4):355–62. This paper provides an extensive validation of organ and tissue scale models, showing their effectiveness for predictive static parameters and indicating the opportunity for better prediction of dynamic parameters.
Manda K, Wallace RJ, Xie SQ, Levrero-Florencio F, Pankaj P. Nonlinear viscoelastic characterization of bovine trabecular bone. Biomech Model Mechanobiol. 2017;16(1):173–89.
Xie SQ, Manda K, Wallace RJ, Levrero-Florencio F, Simpson AHRW, Pankaj P. Time dependent behaviour of trabecular bone at multiple load levels. Ann Biomed Eng. 2017;45(5):1219–26.
Fan Z, Swadener JG, Rho JY, Roy ME, Pharr GM. Anisotropic properties of human tibial cortical bone as measured by nanoindentation. J Orthop Res. 2002;20(4):806–10.
Funding
This work has been supported by the European Union (ERC Advanced MechAGE, ERC-2016-ADG-741883; Marie-Curie-COFUND CaP+MECHLOAD, WHRI-ACADEMY-608765).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ralph Müller reports receiving personal fees from Amgen.
Graeme Paul and Angad Malhotra declare no conflict of interest.
All authors declare no conflicts of interest.
Human and Animal Rights and Informed Consent
This review article does not contain unpublished data from human or animal studies performed by any of the authors.
Additional information
This article is part of the Topical Collection on Biomechanics
Rights and permissions
About this article
Cite this article
Paul, G.R., Malhotra, A. & Müller, R. Mechanical Stimuli in the Local In Vivo Environment in Bone: Computational Approaches Linking Organ-Scale Loads to Cellular Signals. Curr Osteoporos Rep 16, 395–403 (2018). https://doi.org/10.1007/s11914-018-0448-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-018-0448-6