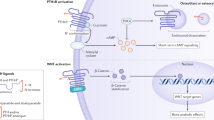
Abstract
Purpose of Review
Numerous forms of osteoporosis in childhood are characterized by low bone turnover (for example, osteoporosis due to neuromuscular disorders and glucocorticoid exposure). Anti-resorptive therapy, traditionally used to treat osteoporosis in the young, is associated with further reductions in bone turnover, raising concerns about the long-term safety and efficacy of such therapy. These observations have led to increasing interest in the role of anabolic therapy to treat pediatric osteoporosis.
Recent Findings
While growth hormone and androgens appears to be relatively weak anabolic modulators of bone mass, emerging therapies targeting bone formation pathways (anti-transforming growth factor beta antibody and anti-sclerostin antibody) hold considerable promise. Teriparatide remains an attractive option that merits formal study for patients post-epiphyseal fusion, although it must be considered that adult studies have shown its effect is blunted when administered following bisphosphonate therapy. Mechanical stimulation of bone through whole body vibration therapy appears to be much less effective than bisphosphonate therapy for treating osteoporosis in children.
Summary
New anabolic therapies which target important pathways in skeletal metabolism merit further study in children, including their effects on fracture risk reduction and after treatment discontinuation.
Similar content being viewed by others
Abbreviations
- bb:
-
Black bear
- BMC:
-
Bone mineral content
- BMD:
-
Bone mineral density
- DXA:
-
Dual energy x-ray absorptiometry
- DMD:
-
Duchenne muscular dystrophy
- GH:
-
Growth hormone
- GHD:
-
Growth hormone deficiency
- OPPG:
-
Osteoporosis pseudoglioma syndrome
- PMO:
-
Post-menopausal osteoporosis
- PTH:
-
Parathyroid hormone
- pQCT:
-
Peripheral quantitative computed tomography
- TGF-beta:
-
Transforming growth factor beta
- WBV:
-
Whole body vibration
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Ward LM, Konji VN, Ma J. The management of osteoporosis in children. Osteoporos Int. 2016;27(7):2147–79.
Trejo P, Rauch F. Osteogenesis imperfecta in children and adolescents-new developments in diagnosis and treatment. Osteoporos Int. 2016;27(12):3427–37.
Misof BM, Roschger P, Klaushofer K, Rauch F, Ma J, Mack DR, et al. Increased bone matrix mineralization in treatment-naive children with inflammatory bowel disease. Bone. 2017;105:50–6.
Sbrocchi AM, Rauch F, Jacob P, McCormick A, McMillan HJ, Matzinger MA, et al. The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporos Int. 2012;23(11):2703–11.
Misof BM, Roschger P, McMillan HJ, Ma J, Klaushofer K, Rauch F, et al. Histomorphometry and bone matrix mineralization before and after bisphosphonate treatment in boys with Duchenne muscular dystrophy: a paired transiliac biopsy study. J Bone Miner Res. 2016;31(5):1060–9.
Rosen T, Hansson T, Granhed H, Szucs J, Bengtsson BA. Reduced bone mineral content in adult patients with growth hormone deficiency. Acta Endocrinol. 1993;129(3):201–6.
Holmes SJ, Economou G, Whitehouse RW, Adams JE, Shalet SM. Reduced bone mineral density in patients with adult onset growth hormone deficiency. J Clin Endocrinol Metab. 1994;78(3):669–74.
Vestergaard P, Jorgensen JO, Hagen C, Hoeck HC, Laurberg P, Rejnmark L, et al. Fracture risk is increased in patients with GH deficiency or untreated prolactinomas—a case-control study. Clin Endocrinol. 2002;56(2):159–67.
Wuster C. Fracture rates in patients with growth hormone deficiency. Horm Res. 2000;54(Suppl 1):31–5.
Cowell CT, Wuster C. The effects of growth hormone deficiency and growth hormone replacement therapy on bone. A meeting report. Horm Res. 2000;54(Suppl 1):68–74.
Schweizer R, Martin DD, Haase M, Roth J, Trebar B, Binder G, et al. Similar effects of long-term exogenous growth hormone (GH) on bone and muscle parameters: a pQCT study of GH-deficient and small-for-gestational-age (SGA) children. Bone. 2007;41(5):875–81.
Hogler W, Briody J, Moore B, Lu PW, Cowell CT. Effect of growth hormone therapy and puberty on bone and body composition in children with idiopathic short stature and growth hormone deficiency. Bone. 2005;37(5):642–50.
Marini JC, Hopkins E, Glorieux FH, Chrousos GP, Reynolds JC, Gundberg CM, et al. Positive linear growth and bone responses to growth hormone treatment in children with types III and IV osteogenesis imperfecta: high predictive value of the carboxyterminal propeptide of type I procollagen. J Bone Miner Res. 2003;18(2):237–43.
Rauch F, Plotkin H, Zeitlin L, Glorieux FH. Bone mass, size, and density in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate therapy. J Bone Miner Res. 2003;18(4):610–4.
Frittoli RB, Longhi BS, Silva AM, Filho AAB, Monteiro M, Appenzeller S. Erratum to “Effects of the use of growth hormone in children, adolescents with juvenile idiopathic arthritis: a systematic review” (Rev Bras Reumatol. 2017;57(2):100-106). Rev Bras Reumatol Engl Ed. 2017;57(5):500.
Frittoli RB, Longhi BS, Silva AM, Filho AAB, Monteiro M, Appenzeller S. Effects of the use of growth hormone in children and adolescents with juvenile idiopathic arthritis: a systematic review. Rev Bras Reumatol Engl Ed. 2017;57(2):100–6.
Ward LM, Kinnett K, Bonewald L. Proceedings of a Parent Project Muscular Dystrophy Bone Health Workshop: morbidity due to osteoporosis in DMD: the path forward May 12–13, 2016, Bethesda, Maryland, USA. Neuromuscul Disord. 2017.
Rutter MM, Collins J, Rose SR, Woo JG, Sucharew H, Sawnani H, et al. Growth hormone treatment in boys with Duchenne muscular dystrophy and glucocorticoid-induced growth failure. Neuromuscul Disord. 2012;22(12):1046–56.
Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, Ellenberg SS, Cauley JA, Ensrud KE, et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial. JAMA Intern Med. 2017;177(4):471–9.
Benito M, Vasilic B, Wehrli FW, Bunker B, Wald M, Gomberg B, et al. Effect of testosterone replacement on trabecular architecture in hypogonadal men. J Bone Miner Res. 2005;20(10):1785–91.
Tracz MJ, Sideras K, Bolona ER, Haddad RM, Kennedy CC, Uraga MV, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 2006;91(6):2011–6.
Reeves PT, Herndon DN, Tanksley JD, Jennings K, Klein GL, Mlcak RP, et al. Five-year outcomes after long-term oxandrolone administration in severely burned children: a randomized clinical trial. Shock. 2016;45(4):367–74.
Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248(3):387–401.
Przkora R, Barrow RE, Jeschke MG, Suman OE, Celis M, Sanford AP, et al. Body composition changes with time in pediatric burn patients. J Trauma. 2006;60(5):968–71. discussion 971
Przkora R, Herndon DN, Suman OE, Jeschke MG, Meyer WJ, Chinkes DL, et al. Beneficial effects of extended growth hormone treatment after hospital discharge in pediatric burn patients. Ann Surg. 2006;243(6):796–801. discussion 801-3
Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract. 2016;22(Suppl 4):1–42.
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41.
Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30(3):312–21.
Vahle JL, Long GG, Sandusky G, Westmore M, Ma YL, Sato M. Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol Pathol. 2004;32(4):426–38.
Lindsay R, Scheele WH, Neer R, Pohl G, Adami S, Mautalen C, et al. Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Intern Med. 2004;164(18):2024–30.
Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, et al. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353(6):555–65.
Ma J, McMillan HJ, Karaguzel G, Goodin C, Wasson J, Matzinger MA, et al. The time to and determinants of first fractures in boys with Duchenne muscular dystrophy. Osteoporos Int. 2017;28(2):597–608.
Catalano A, Vita GL, Russo M, Vita G, Lasco A, Morabito N, et al. Effects of teriparatide on bone mineral density and quality of life in Duchenne muscular dystrophy related osteoporosis: a case report. Osteoporos Int. 2016;27(12):3655–9.
Gray SK, McGee-Lawrence ME, Sanders JL, Condon KW, Tsai CJ, Donahue SW. Black bear parathyroid hormone has greater anabolic effects on trabecular bone in dystrophin-deficient mice than in wild type mice. Bone. 2012;51(3):578–85.
Obermayer-Pietsch BM, Marin F, McCloskey EV, Hadji P, Farrerons J, Boonen S, et al. Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res. 2008;23(10):1591–600.
Beck BR. Vibration therapy to prevent bone loss and falls: mechanisms and efficacy. Curr Osteoporos Rep. 2015;13(6):381–9.
Rauch F, Sievanen H, Boonen S, Cardinale M, Degens H, Felsenberg D, et al. Reporting whole-body vibration intervention studies: recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskelet Neuronal Interact. 2010;10(3):193–8.
• Leonard MB, Shults J, Long J, Baldassano RN, Brown JK, Hommel K, et al. Effect of low-magnitude mechanical stimuli on bone density and structure in pediatric Crohn's disease: a randomized placebo-controlled trial. J Bone Miner Res. 2016;31(6):1177–88. Even though the study outcome is essentially negative, this report provides an excellent template of how to design, conduct, evaluate and report whole-body vibration studies.
Sbrocchi AM, Forget S, Laforte D, Azouz EM, Rodd C. Zoledronic acid for the treatment of osteopenia in pediatric Crohn's disease. Pediatr Int. 2010;52(5):754–61.
Lam TP, Ng BK, Cheung LW, Lee KM, Qin L, Cheng JC. Effect of whole body vibration (WBV) therapy on bone density and bone quality in osteopenic girls with adolescent idiopathic scoliosis: a randomized, controlled trial. Osteoporos Int. 2013;24(5):1623–36.
Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92.
van Lierop AH, Hamdy NA, van Egmond ME, Bakker E, Dikkers FG, Papapoulos SE. Van Buchem disease: clinical, biochemical, and densitometric features of patients and disease carriers. J Bone Miner Res. 2013;28(4):848–54.
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–20.
McColm J, Hu L, Womack T, Tang CC, Chiang AY. Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J Bone Miner Res. 2014;29(4):935–43.
• Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532–43. This randomized, placebo-controlled study examines the effect of romozosumab on women with post-menopausal osteoporosis, showing a significant reduction in all fractures rates but similar adverse effect frequencies.
Chang MK, Kramer I, Keller H, Gooi JH, Collett C, Jenkins D, et al. Reversing LRP5-dependent osteoporosis and SOST deficiency-induced sclerosing bone disorders by altering WNT signaling activity. J Bone Miner Res. 2014;29(1):29–42.
Kedlaya R, Veera S, Horan DJ, Moss RE, Ayturk UM, Jacobsen CM, et al. Sclerostin inhibition reverses skeletal fragility in an Lrp5-deficient mouse model of OPPG syndrome. Sci Transl Med. 2013;5(211):211ra158.
Korvala J, Juppner H, Makitie O, Sochett E, Schnabel D, Mora S, et al. Mutations in LRP5 cause primary osteoporosis without features of OI by reducing Wnt signaling activity. BMC Med Genet. 2012;13:26.
Fahiminiya S, Majewski J, Mort J, Moffatt P, Glorieux FH, Rauch F. Mutations in WNT1 are a cause of osteogenesis imperfecta. J Med Genet. 2013;50(5):345–8.
Palomo T, Glorieux FH, Rauch F. Circulating sclerostin in children and young adults with heritable bone disorders. J Clin Endocrinol Metab. 2014;99(5):E920–5.
Sinder BP, Eddy MM, Ominsky MS, Caird MS, Marini JC, Kozloff KM. Sclerostin antibody improves skeletal parameters in a Brtl/+ mouse model of osteogenesis imperfecta. J Bone Miner Res. 2013;28(1):73–80.
Jacobsen CM, Barber LA, Ayturk UM, Roberts HJ, Deal LE, Schwartz MA, et al. Targeting the LRP5 pathway improves bone properties in a mouse model of osteogenesis imperfecta. J Bone Miner Res. 2014;29(10):2297–306.
Grafe I, Alexander S, Yang T, Lietman C, Homan EP, Munivez E, et al. Sclerostin antibody treatment improves the bone phenotype of Crtap(−/−) mice, a model of recessive osteogenesis imperfecta. J Bone Miner Res. 2016;31(5):1030–40.
Roschger A, Roschger P, Keplingter P, Klaushofer K, Abdullah S, Kneissel M, et al. Effect of sclerostin antibody treatment in a mouse model of severe osteogenesis imperfecta. Bone. 2014;66:182–8.
Rauch F, Lalic L, Roughley P, Glorieux FH. Relationship between genotype and skeletal phenotype in children and adolescents with osteogenesis imperfecta. J Bone Miner Res. 2010;25(6):1367–74.
Chen XX, Baum W, Dwyer D, Stock M, Schwabe K, Ke HZ, et al. Sclerostin inhibition reverses systemic, periarticular and local bone loss in arthritis. Ann Rheum Dis. 2013;72(10):1732–6.
Eddleston A, Marenzana M, Moore AR, Stephens P, Muzylak M, Marshall D, et al. A short treatment with an antibody to sclerostin can inhibit bone loss in an ongoing model of colitis. J Bone Miner Res. 2009;24(10):1662–71.
Marenzana M, Greenslade K, Eddleston A, Okoye R, Marshall D, Moore A, et al. Sclerostin antibody treatment enhances bone strength but does not prevent growth retardation in young mice treated with dexamethasone. Arthritis Rheum. 2011;63(8):2385–95.
Cui L, Cheng H, Song C, Li C, Simonet WS, Ke HZ, et al. Time-dependent effects of sclerostin antibody on a mouse fracture healing model. J Musculoskelet Neuronal Interact. 2013;13(2):178–84.
Makhdom AM, Rauch F, Lauzier D, Hamdy RC. The effect of systemic administration of sclerostin antibody in a mouse model of distraction osteogenesis. J Musculoskelet Neuronal Interact. 2014;14(1):124–30.
• Wehmeyer C, Frank S, Beckmann D, Bottcher M, Cromme C, Konig U, et al. Sclerostin inhibition promotes TNF-dependent inflammatory joint destruction. Sci Transl Med. 2016;8(330):330ra35. Sclerostin is generally thought to be a bone-specific protein, but this study shows that in pathological situations anti-sclerostin treatments may have unexpected adverse effects. In a mouse model of rheumatoid arthritis, sclerostin inhibition led to increased cartilage destruction.
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377(15):1417–27.
Perosky JE, Khoury BM, Jenks TN, Ward FS, Cortright K, Meyer B, et al. Single dose of bisphosphonate preserves gains in bone mass following cessation of sclerostin antibody in Brtl/+ osteogenesis imperfecta model. Bone. 2016;93:79–85.
Recknor CP, Recker RR, Benson CT, Robins DA, Chiang AY, Alam J, et al. The effect of discontinuing treatment with blosozumab: follow-up results of a phase 2 randomized clinical trial in postmenopausal women with low bone mineral density. J Bone Miner Res. 2015;30(9):1717–25.
Janssens K, Vanhoenacker F, Bonduelle M, Verbruggen L, Van Maldergem L, Ralston S, et al. Camurati-Engelmann disease: review of the clinical, radiological, and molecular data of 24 families and implications for diagnosis and treatment. J Med Genet. 2006;43(1):1–11.
Verstraeten A, Alaerts M, Van Laer L, Loeys B. Marfan syndrome and related disorders: 25 years of gene discovery. Hum Mutat. 2016;37(6):524–31.
• Grafe I, Yang T, Alexander S, Homan EP, Lietman C, Jiang MM, et al. Excessive transforming growth factor-beta signaling is a common mechanism in osteogenesis imperfecta. Nat Med. 2014;20(6):670–5. This study shows that altered TGFβ matrix-cell signaling is a primary mechanism in the pathogenesis of OI, and could be a promising target for the treatment of OI.
Loeys BL. Angiotensin receptor blockers: a panacea for Marfan syndrome and related disorders? Drug Discov Today. 2015;20(2):262–6.
Ayyavoo A, Derraik JG, Cutfield WS, Hofman PL. Elimination of pain and improvement of exercise capacity in Camurati-Engelmann disease with losartan. J Clin Endocrinol Metab. 2014;99(11):3978–82.
Trifiro G, Marelli S, Viecca M, Mora S, Pini A. Areal bone mineral density in children and adolescents with Marfan syndrome: evidence of an evolving problem. Bone. 2015;73:176–80.
Acknowledgements
Dr. Ward is supported by a Research Chair Award from the University of Ottawa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Leanne Ward is participating in clinical trials with AMGEN, outside the submitted work. Frank Rauch declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pediatrics
Rights and permissions
About this article
Cite this article
Ward, L.M., Rauch, F. Anabolic Therapy for the Treatment of Osteoporosis in Childhood. Curr Osteoporos Rep 16, 269–276 (2018). https://doi.org/10.1007/s11914-018-0434-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-018-0434-z