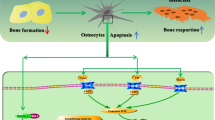
Abstract
Studies from the 1950s and 1960s already recognize the fact that osteocytes, although long living cells, die, as evidenced by accumulation of osteocytic lacunae devoid of cells. More recently, it was demonstrated that these cells die by apoptosis. The rate of osteocyte apoptosis is regulated by the age of the bone, as well as by systemic hormones, local growth factors, cytokines, pharmacological agents, and mechanical forces. Apoptotic osteocytes, in turn, recruit osteoclasts to initiate targeted bone resorption. This results in the removal of “dead” bone and may improve the mechanical properties of the skeleton. However, the molecular regulators of osteocyte survival and targeted bone remodeling are not completely known. In this review, the current knowledge on the molecular mechanism that lead to osteocyte death or survival, and the signals that mediate targeted bone resorption is discussed.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bellido T. Osteocyte-driven bone remodeling. Calcif Tissue Int. 2013;94:25–34.
Noble BS, Reeve J. Osteocyte function, osteocyte death and bone fracture resistance. Mol Cell Endocrinol. 2000;159:7–13.
Frost HM. In vivo osteocyte death. J Bone Joint Surg Am. 1960;42-A:138–43.
Almeida M, Han L, Martin-Millan M, et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–97.
Tomkinson A, Reeve J, Shaw RW, et al. The death of osteocytes via apoptosis accompanies estrogen withdrawal in human bone. J Clin Endocrinol Metab. 1997;82:3128–35.
Kousteni S, Bellido T, Plotkin LI, et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–30.
Weinstein RS, Jilka RL, Parfitt AM, et al. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–82.
Aguirre JI, Plotkin LI, Stewart SA, et al. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res. 2006;21:605–15.
Morse LR, Xu Y, Solomon B, et al. Severe spinal cord injury causes immediate multi-cellular dysfunction at the chondro-osseous junction. Transl Stroke Res. 2011;2:643–50.
Verborgt O, Gibson G, Schaffler MB. Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res. 2000;15:60–7.
Jilka RL, Weinstein RS, Bellido T, et al. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest. 1999;104:439–46.
Plotkin LI, Weinstein RS, Parfitt AM, et al. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–74.
Gohel A, McCarthy MB, Gronowicz G. Estrogen prevents glucocorticoid-induced apoptosis in osteoblasts in vivo and in vitro. Endocrinology. 1999;140:5339–47.
Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab. 2010;21:369–74.
Jilka RL, Bellido T, Almeida M, Plotkin LI, O'Brien CA, Weinstein RS, et al. Apoptosis in bone cells. In: Bilezikian JP, Raisz LG, Martin TJ, editors. Principles of bone biology. San Diego, San Francisco, New York, London, Sydney, Tokyo: Academic Press; 2008. p. 237–61.
Jilka RL, O'Brien CA, Roberson PK, et al. Dysapoptosis of osteoblasts and osteocytes increases cancellous bone formation but exaggerates bone porosity with age. J Bone Miner Res. 2014;29:103–17. Reports the effect of preventing osteocyte apoptosis on gene expression and bone remodeling.
Weinstein RS, Nicholas RW, Manolagas SC. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J Clin Endocrinol Metab. 2000;85:2907–12.
Plotkin LI, Manolagas SC, Bellido T. Glucocorticoids induce osteocyte apoptosis by blocking focal adhesion kinase-mediated survival: evidence for inside-out signaling leading to anoikis. J Biol Chem. 2007;282:24120–30.
Canalis E, Delany AM. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci. 2002;966:73–81.
Wang FS, Lin CL, Chen YJ, et al. Secreted frizzled-related protein 1 (SFRP1) modulates glucocorticoid attenuation of osteogenic activities and bone mass. Endocrinology. 2005;146:2415–23.
Almeida M, Han L, Ambrogini E, et al. Glucocorticoids and tumor necrosis factor (TNF) alpha increase oxidative stress and suppress WNT signaling in osteoblasts. J Biol Chem. 2011;286:44326–35.
Jia J, Yao W, Guan M, et al. Glucocorticoid dose determines osteocyte cell fate. FASEB J. 2011;25:3366–76.
Weinstein RS, Wan C, Liu Q, et al. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in 21-month-old mice. Aging Cell. 2009;9:147–61.
Noble BS, Peet N, Stevens HY, et al. Mechanical loading: biphasic osteocyte survival and the targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol. 2003;284:C934–43.
Lin C, Jiang X, Dai Z, et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res. 2009;24:1651–61.
Verborgt O, Tatton NA, Majeska RJ, et al. Spatial distribution of Bax and Bcl-2 in osteocytes after bone fatigue: complementary roles in bone remodeling regulation? J Bone Miner Res. 2002;17:907–14.
Bellido T, Ali AA, Plotkin LI, et al. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278:50259–72.
Bivi N, Lezcano V, Romanello M, et al. Connexin43 interacts with barrestin: a prerequisite for osteoblast survival induced by parathyroid hormone. J Cell Biochem. 2011;112:2920–30.
Rogers MJ, Crockett JC, Coxon FP, et al. Biochemical and molecular mechanisms of action of bisphosphonates. Bone. 2011;49:34–41.
Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem. 2002;277:8648–57.
Plotkin LI, Lezcano V, Thostenson J, et al. Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. J Bone Miner Res. 2008;23:1712–21.
Plotkin LI, Bivi N, Bellido T. A bisphosphonate that does not affect osteoclasts prevents osteoblast and osteocyte apoptosis and the loss of bone strength induced by glucocorticoids in mice. Bone. 2011;49:122–7.
Plotkin LI, Aguirre JI, Kousteni S, et al. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of ERK activation. J Biol Chem. 2005;280:7317–25.
Plotkin LI, Manolagas SC, Bellido T. Dissociation of the pro-apoptotic effects of bisphosphonates on osteoclasts from their anti-apoptotic effects on osteoblasts/osteocytes with novel analogs. Bone. 2006;39:443–52.
Tomkinson A, Gevers EF, Wit JM, et al. The role of estrogen in the control of rat osteocyte apoptosis. J Bone Miner Res. 1998;13:1243–50.
Kousteni S, Chen JR, Bellido T, et al. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298:843–6.
Huber C, Collishaw S, Mosley JR, et al. Selective estrogen receptor modulator inhibits osteocyte apoptosis during abrupt estrogen withdrawal: implications for bone quality maintenance. Calcif Tissue Int. 2007;81:139–44.
Kousteni S, Han L, Chen JR, et al. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest. 2003;111:1651–64.
van Essen HW, Holzmann PJ, Blankenstein MA, et al. Effect of raloxifene treatment on osteocyte apoptosis in postmenopausal women. Calcif Tissue Int. 2007;81:183–90.
Plotkin LI, Mathov I, Aguirre JI, et al. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins, Src kinases, and ERKs. Am J Physiol Cell Physiol. 2005;289:C633–43.
Aguirre JI, Plotkin LI, Gortazar AR, et al. A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J Biol Chem. 2007;282:25501–8.
Kitase Y, Barragan L, Jiang JX, et al. Mechanical induction of PGE(2) in osteocytes blocks glucocorticoid induced apoptosis through both the beta-catenin and PKA pathways. J Bone Miner Res. 2010;25:2657–68.
Frost HM. Bone's mechanostat: a 2003 update. Anat Rec. 2003;275A:1081–101.
Noble BS, Stevens H, Loveridge N, et al. Identification of apoptotic changes in osteocytes in normal and pathological human bone. Bone. 1997;20:273–82.
Elmardi AS, Katchburian MV, Katchburian E. Electron microscopy of developing calvaria reveals images that suggest that osteoclasts engulf and destroy osteocytes during bone resorption. Calcif Tissue Int. 1990;46:239–45.
Boabaid F, Cerri PS, Katchburian E. Apoptotic bone cells may be engulfed by osteoclasts during alveolar bone resorption in young rats. Tissue Cell. 2001;33:318–25.
Cerri PS, Boabaid F, Katchburian E. Combined TUNEL and TRAP methods suggest that apoptotic bone cells are inside vacuoles of alveolar bone osteoclasts in young rats. J Periodontal Res. 2003;38:223–6.
Cardoso L, Herman BC, Verborgt O, et al. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res. 2009;24:597–605.
Emerton KB, Hu B, Woo AA, et al. Osteocyte apoptosis and control of bone resorption following ovariectomy in mice. Bone. 2009;46:577–83.
Tatsumi S, Ishii K, Amizuka N, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5:464–75.
Bivi N, Condon KW, Allen MR, et al. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Miner Res. 2012;27:374–89.
Lloyd SA, Loiselle AE, Zhang Y, et al. Connexin 43 deficiency desensitizes bone to the effects of mechanical unloading through modulation of both arms of bone remodeling. Bone. 2013;57:76–83.
Schaffler MB, Cheung WY, Majeska R, et al. Osteocytes: master orchestrators of bone. Calcif Tissue Int. 2013;94:5–24.
Kennedy OD, Herman BC, Laudier DM, et al. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone. 2012;50:1115–22. Reports the temporal relationships between injury, osteocyte apoptosis, and pro-osteoclastogenic signaling following excess loading.
Wu AC, Morrison NA, Kelly WL, et al. MCP-1 expression is specifically regulated during activation of skeletal repair and remodeling. Calcif Tissue Int. 2013;92:566–75.
Zhang Y, Paul EM, Sathyendra V, et al. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS One. 2011;6:e23516.
Kogianni G, Mann V, Noble BS. Apoptotic bodies convey activity capable of initiating osteoclastogenesis and localized bone destruction. J Bone Miner Res. 2008;23:915–27.
Yang J, Shah R, Robling AG, et al. HMGB1 is a bone-active cytokine. J Cell Physiol. 2008;214:730–9.
Klune JR, Dhupar R, Cardinal J, et al. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–84.
Zhou Z, Han JY, Xi CX, et al. HMGB1 regulates RANKL-induced osteoclastogenesis in a manner dependent on RAGE. J Bone Miner Res. 2008;23:1084–96.
Taniguchi N, Yoshida K, Ito T, et al. Stage-specific secretion of HMGB1 in cartilage regulates endochondral ossification. Mol Cell Biol. 2007;27:5650–63.
Acknowledgement
This research was supported by National Institutes of Health (R01-AR053643) and by a Biomedical Research Grant and a Developing Diverse Researchers with InVestigative Expertise (DRIVE) Grant from Indiana University School of Medicine.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
L.I. Plotkin declares that she has no conflicts of interest.
Human and Animal Rights and Informed Consent
All studies by L. I. Plotkin involving murine samples were performed after approval by the Institutional Animal Care and Use Committees of University of Arkansas for Medical Sciences and Indiana University School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Plotkin, L.I. Apoptotic Osteocytes and the Control of Targeted Bone Resorption. Curr Osteoporos Rep 12, 121–126 (2014). https://doi.org/10.1007/s11914-014-0194-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-014-0194-3